当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In silico identification and in vitro evaluation of a protein-protein interaction inhibitor of Escherichia coli fatty acid biosynthesis
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2021-04-27 , DOI: 10.1111/cbdd.13851 Katherine Charov 1 , Michael D Burkart 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2021-04-27 , DOI: 10.1111/cbdd.13851 Katherine Charov 1 , Michael D Burkart 1
Affiliation
|
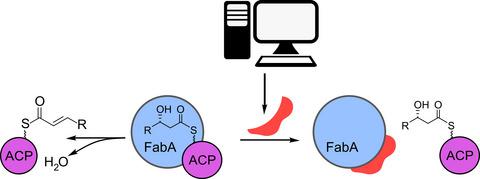
To combat the rise in antibiotic resistance, new targets must be identified and probes against them developed. Protein-protein interactions (PPI) of bacterial type II fatty acid biosynthesis (FAS-II) represent an untapped, yet rich area for new antibiotic discovery. Here, we present a computational and in vitro workflow for the discovery of new inhibitors of PPI in Escherichia coli FAS-II. As part of this study, we identified suramin, an existing treatment for African sleeping sickness, to effectively block the interaction of E. coli dehydratase FabA and the acyl carrier protein EcACP, with an IC50 = 85 μΜ. This finding validates a workflow that combines in silico screening with in vitro PPI assays to identify probes appropriate for further optimization.
中文翻译:

大肠杆菌脂肪酸生物合成的蛋白质-蛋白质相互作用抑制剂的计算机识别和体外评价
为了对抗抗生素耐药性的上升,必须确定新的目标并开发针对它们的探针。细菌 II 型脂肪酸生物合成 (FAS-II) 的蛋白质-蛋白质相互作用 (PPI) 代表了一个尚未开发但丰富的新抗生素发现领域。在这里,我们提出了一个计算和体外工作流程,用于在大肠杆菌FAS-II 中发现新的 PPI 抑制剂。作为这项研究的一部分,我们确定了苏拉明(一种用于治疗非洲昏睡病的现有疗法)可有效阻断大肠杆菌脱水酶 FabA 与酰基载体蛋白 EcACP 的相互作用,IC 50 = 85 μM。这一发现验证了将计算机筛选与体外 PPI 分析相结合的工作流程,以确定适合进一步优化的探针。
更新日期:2021-06-21
中文翻译:

大肠杆菌脂肪酸生物合成的蛋白质-蛋白质相互作用抑制剂的计算机识别和体外评价
为了对抗抗生素耐药性的上升,必须确定新的目标并开发针对它们的探针。细菌 II 型脂肪酸生物合成 (FAS-II) 的蛋白质-蛋白质相互作用 (PPI) 代表了一个尚未开发但丰富的新抗生素发现领域。在这里,我们提出了一个计算和体外工作流程,用于在大肠杆菌FAS-II 中发现新的 PPI 抑制剂。作为这项研究的一部分,我们确定了苏拉明(一种用于治疗非洲昏睡病的现有疗法)可有效阻断大肠杆菌脱水酶 FabA 与酰基载体蛋白 EcACP 的相互作用,IC 50 = 85 μM。这一发现验证了将计算机筛选与体外 PPI 分析相结合的工作流程,以确定适合进一步优化的探针。



























 京公网安备 11010802027423号
京公网安备 11010802027423号