Atmospheric Environment ( IF 5 ) Pub Date : 2021-04-25 , DOI: 10.1016/j.atmosenv.2021.118436 Tingting Xie , Senlin Lu , Lanfang Rao , Luying Zhang , Xingzi Wang , Weiqian Wang , Qingyue Wang
|
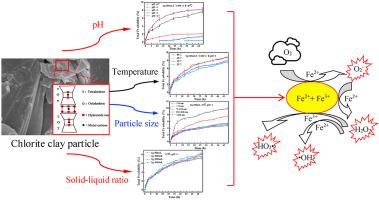
Soluble irons from aerosol particles play a key role in assessment of biological and toxicological effects as a result of their oxidative potential. Several factors are responsible for controlling the solubility of irons released from aerosol mineral particles in acid solution. Here, factors of H+ concentrations, temperatures, solid–liquid ratios and particle sizes on acidsoluble irons from chlorite mineral particles were investigated in 48h. Our data demonstrated that the higher acidity, higher temperature, lower solid–liquid ratio, and smaller particle size were factors that positively influenced the solubility of FeT and Fe(II), and that the higher acidity, lower temperature, higher solid–liquid ratio, and smaller particle size positively improved the solubility of Fe(III). Fe(II) dissolved more easily than Fe(III) in the acid solution under all conditions; the percentage of Fe(III) among the total iron released from chlorite particles could be promoted by increasing the acidity, solid–liquid ratio, and particle size, or by lowering the temperature. In this acidolysis process, there is a clear order of importance of these influencing factors, H+ concentrations > particle sizes > temperatures and solid–liquid ratios. In addition, the dissolution data for FeT at pH = 0.7 and pH = 1 in 48 h could be well described and predicted by non-linear fitting with r2 > 0.99 according to continuous dissolution model. And the analysis of oxidative potential showed that Fe(II) dissolved from chlorite possessed a dominant position in generating reactive oxygen species.
中文翻译:

亚氯酸盐矿物颗粒中酸溶性铁的溶解因子和氧化电位
气溶胶颗粒中的可溶性铁由于具有氧化潜力,因此在评估生物学和毒理学效应方面起着关键作用。有几个因素负责控制从气溶胶矿物颗粒释放的铁在酸性溶液中的溶解度。在这里,在48小时内研究了来自亚氯酸盐矿物颗粒的酸溶性铁的H +浓度,温度,固液比和粒径的因素。我们的数据表明,较高的酸度,较高的温度,较低的固液比和较小的粒径是积极影响Fe T溶解度的因素和Fe(II),较高的酸度,较低的温度,较高的固液比和较小的粒径可积极改善Fe(III)的溶解度。在所有条件下,Fe(II)都比Fe(III)更容易溶解在酸性溶液中;通过增加酸度,固液比和粒径,或降低温度,可以提高亚氯酸盐颗粒释放的总铁中Fe(III)的百分比。在这种酸解过程中,这些影响因素的重要性具有明显的顺序,即H +浓度>粒径>温度和固液比。此外,可以通过描述r 2的非线性拟合,很好地描述和预测Fe T在pH = 0.7和pH = 1下在48小时内的溶出度数据。 > 0.99(根据连续溶出模型)。氧化电位的分析表明,从亚氯酸盐中溶解的Fe(II)在产生活性氧方面具有主导地位。



























 京公网安备 11010802027423号
京公网安备 11010802027423号