当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Measurements and Thermodynamic Modeling of Liquid–Liquid Equilibrium Data for 2-Methyl-2-Butanol + Water + Ethylbenzene
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-04-23 , DOI: 10.1021/acs.jced.1c00056 Xuemei Zhang 1 , Chungui Jian 2 , Jianting Du 2 , Hongwei Wang 3 , Wangxin Kong 3 , Junwei Chen 3 , Guangjin Shangguan 3 , Meifang Xia 3 , Huangpeng Lu 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-04-23 , DOI: 10.1021/acs.jced.1c00056 Xuemei Zhang 1 , Chungui Jian 2 , Jianting Du 2 , Hongwei Wang 3 , Wangxin Kong 3 , Junwei Chen 3 , Guangjin Shangguan 3 , Meifang Xia 3 , Huangpeng Lu 3
Affiliation
|
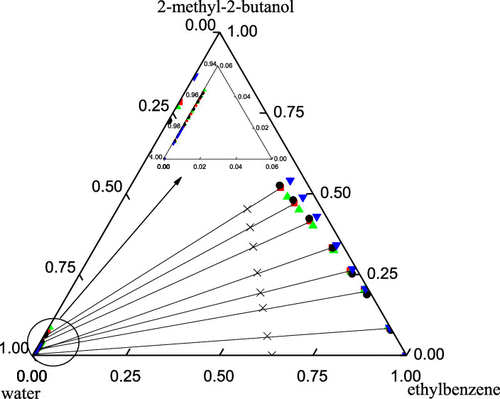
Liquid–liquid equilibrium (LLE) data for the mixture 2-methyl-2-butanol + water + ethylbenzene were measured at 303.15, 313.15, and 323.15 K at atmospheric pressure. The quality of the experimental LLE data was tested by the mass balance between the feed and the conjugated phases. The extraction capacity of ethylbenzene was evaluated by selectivity (S). The experimental results show that S is more than 117, and it decreases with the content of 2-methyl-2-butanol in the water phase and equilibrium temperature. The binary interaction parameters for the NRTL and UNIQUAC models were derived from regression of the experimental LLE data. The root-mean-square deviations are no more than 1.27%. The consistency between the regressed binary interaction parameters and the experimental LLE data was verified using the topology of Gibbs energy of mixing surface analysis.
中文翻译:

2-甲基-2-丁醇+水+乙苯的液-液平衡数据的测量和热力学建模
在大气压下,在303.15、313.15和323.15 K下测量了2-甲基-2-丁醇+水+乙苯混合物的液-液平衡(LLE)数据。通过进料和共轭相之间的质量平衡测试了实验性LEE数据的质量。通过选择性(S)评估乙苯的萃取能力。实验结果表明,小号大于117,并且随着水相中2-甲基-2-丁醇的含量和平衡温度而降低。NRTL和UNIQUAC模型的二元相互作用参数来自实验LLE数据的回归。均方根偏差不超过1.27%。使用混合表面分析的吉布斯能量拓扑结构,验证了回归二元相互作用参数与实验LLE数据之间的一致性。
更新日期:2021-05-13
中文翻译:

2-甲基-2-丁醇+水+乙苯的液-液平衡数据的测量和热力学建模
在大气压下,在303.15、313.15和323.15 K下测量了2-甲基-2-丁醇+水+乙苯混合物的液-液平衡(LLE)数据。通过进料和共轭相之间的质量平衡测试了实验性LEE数据的质量。通过选择性(S)评估乙苯的萃取能力。实验结果表明,小号大于117,并且随着水相中2-甲基-2-丁醇的含量和平衡温度而降低。NRTL和UNIQUAC模型的二元相互作用参数来自实验LLE数据的回归。均方根偏差不超过1.27%。使用混合表面分析的吉布斯能量拓扑结构,验证了回归二元相互作用参数与实验LLE数据之间的一致性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号