当前位置:
X-MOL 学术
›
ACS Food Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating the Interaction Mechanism of Folic Acid with Ovalbumin by Multispectroscopic and Molecular Simulation Methods
ACS Food Science & Technology Pub Date : 2021-04-19 , DOI: 10.1021/acsfoodscitech.1c00017 Junyi Wang 1 , Younas Dadmohammadi 1 , Archana Jaiswal 2 , Alireza Abbaspourrad 1
ACS Food Science & Technology Pub Date : 2021-04-19 , DOI: 10.1021/acsfoodscitech.1c00017 Junyi Wang 1 , Younas Dadmohammadi 1 , Archana Jaiswal 2 , Alireza Abbaspourrad 1
Affiliation
|
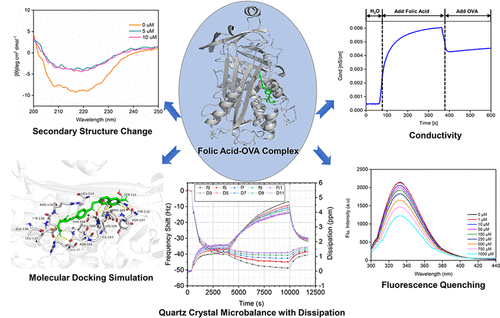
Pharmaceutical and food applications of folic acid are restrained by its stability and solubility. Different techniques, such as complexation or encapsulation, have been reported to improve stability and solubility. Thus, the understanding of folic acid interaction mechanism with macromolecules like ovalbumin (OVA) is a crucial step. This study characterized the folic acid–OVA interactions using fluorescence titration, FTIR, circular dichroism (CD), quartz-crystal microbalance with dissipation (QCM-D), and molecular docking. Fluorescence titration showed that folic acid could strongly quench the intrinsic fluorescence of OVA through static quenching. The thermodynamic parameters, ΔH (−35.06 kJ mol–1), ΔS (−32.27 J mol–1 K–1 at 298 K), and ΔG (−27.98 kJ mol–1 at 298 K), revealed that the folic acid–OVA interaction was mainly driven by hydrogen bonding. Molecular docking and QCM-D indicated folic acid could be inserted into the OVA hydrophobic cavity between α-helical and β-sheet-rich domains. This altered OVA conformation by reducing α-helix and increasing β-sheets, proved by CD and FTIR. Furthermore, when the OVA was used as a carrier, the solubility of folic acid increased by 15-fold to 84.05 μg/mL compared to that of free folic acid.
中文翻译:

用多光谱和分子模拟方法阐明叶酸与卵清蛋白的相互作用机理
叶酸的稳定性和溶解性限制了其在制药和食品中的应用。已经报道了不同的技术,例如络合或封装,以提高稳定性和溶解性。因此,了解叶酸与大分子卵清蛋白(OVA)相互作用的机制是至关重要的一步。这项研究使用荧光滴定,FTIR,圆二色性(CD),带耗散的石英晶体微天平(QCM-D)和分子对接来表征叶酸与OVA的相互作用。荧光滴定表明,叶酸可以通过静态猝灭来强烈猝灭OVA的内在荧光。热力学参数ΔH(−35.06 kJ mol –1),ΔS(−32.27 J mol –1 K在298 K时为–1)和ΔG(在298 K时为-27.98 kJ mol –1)表明,叶酸与OVA的相互作用主要是由氢键驱动的。分子对接和QCM-D表明叶酸可以插入到富含α-螺旋结构域和β-折叠结构域之间的OVA疏水腔中。CD和FTIR证明,这可通过减少α-螺旋和增加β-折叠来改变OVA构象。此外,当将OVA用作载体时,叶酸的溶解度与游离叶酸的溶解度相比增加了15倍,达到84.05μg/ mL。
更新日期:2021-05-22
中文翻译:

用多光谱和分子模拟方法阐明叶酸与卵清蛋白的相互作用机理
叶酸的稳定性和溶解性限制了其在制药和食品中的应用。已经报道了不同的技术,例如络合或封装,以提高稳定性和溶解性。因此,了解叶酸与大分子卵清蛋白(OVA)相互作用的机制是至关重要的一步。这项研究使用荧光滴定,FTIR,圆二色性(CD),带耗散的石英晶体微天平(QCM-D)和分子对接来表征叶酸与OVA的相互作用。荧光滴定表明,叶酸可以通过静态猝灭来强烈猝灭OVA的内在荧光。热力学参数ΔH(−35.06 kJ mol –1),ΔS(−32.27 J mol –1 K在298 K时为–1)和ΔG(在298 K时为-27.98 kJ mol –1)表明,叶酸与OVA的相互作用主要是由氢键驱动的。分子对接和QCM-D表明叶酸可以插入到富含α-螺旋结构域和β-折叠结构域之间的OVA疏水腔中。CD和FTIR证明,这可通过减少α-螺旋和增加β-折叠来改变OVA构象。此外,当将OVA用作载体时,叶酸的溶解度与游离叶酸的溶解度相比增加了15倍,达到84.05μg/ mL。


























 京公网安备 11010802027423号
京公网安备 11010802027423号