Letters in Drug Design & Discovery ( IF 1 ) Pub Date : 2021-02-28 , DOI: 10.2174/1570180817999201016154034 Yıldız Uygun Cebeci 1 , Sule Ceylan 2 , Neslihan Demirbas 1 , Şengül Alpay Karaoğlu 3
|
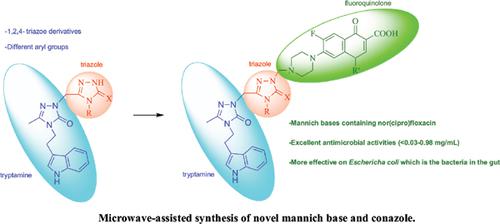
Background: The aim of this study was to synthesize new mannich bases and conazol derivatives with biological activity by the microwave-assisted method.
Introduction: 1,2,4-Triazole-3-one (3) acquired from tryptamine was transformed to the corresponding carbox(thio)amides (6a-c) via several steps. Compounds 6a-c were refluxed with sodium hydroxide to yield 1,2,4-triazole derivatives (7a-c). Compounds 3 and 7a-c on treatment with different heterocyclic secondary amines in an ambiance with formaldehyde afforded the mannich bases 8-15 having diverse pharmacophore units with biologically active sites. The reaction of compound 3 and 2-bromo-1-(4-chlorophenyl) ethanone in the presence of sodium ethoxide gave the corresponding product 2-substituted-1,2,4-triazole-3-one, 16, which was reduced to 1,2,4-triazoles (17). Synthesis of compounds 18, 19, and 20 was carried out starting from compounds 17 with 4-chlorobenzyl chloride (for 18), 2,4-dichlorobenzyl chloride (for 19), and 2,6-dichlorobenzyl chloride (for 20).
Methods: The conventional technique was utilized for the synthesis of compounds, 3-7, and microwave- assisted technique for the compounds, 8-20. That is, green chemistry techniques were applied during these reactions. The structures of molecules were elucidated on the foundation of 1H NMR, 13C NMR, FT-IR, EI-MS methods, and elemental analysis. Novel synthesized molecules were investigated for their antimicrobial activity using MIC (minimum inhibitory concentration) method.
Results: Aminoalkylation of triazole derivatives 3 and 7a-c with fluoroquinolones such as ciprofloxacin and norfloxacin provided an enhancement to the bioactivity of mannich bases 8-11 against the tested microorganisms. The MIC values ranged between <0.24 and 3.9 μg/mL. Moreover, molecules 10 and 11 exhibited more effects on M. smegmatis than the other compounds by the MIC values of <1 μg/mL. They have shown very good antituberculosis activity.
Conclusion: Most of the synthesized structures were observed to have excellent antimicrobial activity against most microorganisms taken into account. These molecules have better activity than the standard drug ampicillin and streptomycin.
中文翻译:

微波合成含有生物活性药理基团的新型曼尼希碱和康唑衍生物
背景:本研究的目的是通过微波辅助方法合成具有生物活性的新的曼尼希碱和康那唑衍生物。
简介:通过多种步骤,将从色胺中获得的1,2,4-三唑-3-酮(3)转化为相应的羧(硫)酰胺(6a-c)。将化合物6a-c与氢氧化钠回流,得到1,2,4-三唑衍生物(7a-c)。在甲醛气氛下用不同的杂环仲胺处理化合物3和7a-c,得到具有具有生物活性位点的多种药效团单元的曼尼希碱8-15。化合物3与2-溴-1-(4-氯苯基)乙酮在乙醇钠存在下的反应得到相应的产物2-取代-1,2,4-三唑-3-酮16,将其还原为1,2,4-三唑(17)。从化合物17开始,用4-氯苄基氯(对于18),2,4-二氯苄基氯(对于19)和2,进行化合物18、19和20的合成。
方法:采用常规技术合成化合物3-7,采用微波辅助技术合成化合物8-20。也就是说,在这些反应过程中应用了绿色化学技术。在1 H NMR,13 C NMR,FT-IR,EI-MS方法和元素分析的基础上阐明了分子的结构。使用MIC(最小抑菌浓度)方法研究了新型合成分子的抗菌活性。
结果:用氟喹诺酮类如环丙沙星和诺氟沙星对三唑衍生物3和7a-c进行氨烷基化可增强曼尼希氏碱8-11对被测微生物的生物活性。MIC值介于<0.24和3.9μg/ mL之间。而且,通过<1μg/ mL的MIC值,分子10和11对耻垢分枝杆菌显示出比其他化合物更大的作用。他们显示出非常好的抗结核活性。
结论:考虑到大多数合成的结构对大多数微生物具有优异的抗菌活性。这些分子比标准药物氨苄西林和链霉素具有更好的活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号