Environmental Technology & Innovation ( IF 7.1 ) Pub Date : 2021-04-18 , DOI: 10.1016/j.eti.2021.101564 Samira Sheikhi , Reza Dehghanzadeh , Ammar Maryamabadi , Hassan Aslani
|
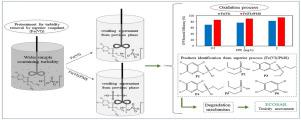
Chlorpyrifos (CPF), as a highly toxic pesticide, is extensively consumed in the agricultural industry and may cause environmental damage. The aim of present study was to evaluate the potential usage of Fe(VI) and Fe(VI)/PMS processes for CPF oxidation in an aqueous solution. The study was conducted in two phases including coagulation and flocculation, as well as advanced oxidation process (AOP). In the first phase, the coagulation process, as a prevalent pretreatment for AOP, was performed for turbidity removal by two types of coagulants, FeO 4 and FeCl 3. FeO 4 showed the best performance as a coagulant at acidic pH=3.0 with an efficiency of 97.47%, while FeCl 3 revealed the highest efficiency (95.79%) at alkaline pH=8.01. However, only the supernatant from the Fe(VI) coagulation process was selected for the next phase analysis. In the next phase (AOP), the results showed that the removal efficiency of Fe(VI)/PMS system was higher than that of Fe(VI) process within the entire pH range. Under optimum conditions ([Fe(VI)]:[PMS]=5:1, pH=7.0, and CPF=2 mg/L), 94.75% removal efficiency was achieved by Fe(VI)/PMS, while it was 82.80% for Fe(VI) alone. The CPF degradation pathways using Fe(VI)/PMS process were proposed, including the P=S bond oxidation, the C–O bond cleavage, diethylation as well as reaction of hydroxyl substitution, leading to the generation of six products. The ECOSAR simulation program showed that the chronic and acute toxicity of CPF degradation byproducts in the Fe(VI)/PMS system relatively decreased. In summary, the study indicated that the Fe(VI)/PMS oxidation is a compatible and efficient technique for removing CPF from aqueous media.
中文翻译:

通过顺序使用混凝和高级氧化工艺从水溶液中去除毒死rif:副产物,降解途径和毒性评估
毒死rif(CPF)是一种剧毒农药,在农业中被大量消费,并可能造成环境破坏。本研究的目的是评估在水溶液中进行CPF氧化的Fe(VI)和Fe(VI)/ PMS工艺的潜在用途。该研究分两个阶段进行,包括凝结和絮凝以及高级氧化工艺(AOP)。在第一阶段中,作为AOP的一种普遍预处理方法,进行了混凝工艺,以通过两种类型的混凝剂去除混浊,FeO 4和FeCl 3。FeO 4在酸性pH = 3.0时表现出最佳的凝结性能,效率为97.47%,而FeCl 3揭示了在碱性pH = 8.01时最高的效率(95.79%)。但是,只有来自Fe(VI)凝聚过程的上清液才被选择用于下一阶段分析。在下一阶段(AOP)中,结果表明,在整个pH范围内,Fe(VI)/ PMS系统的去除效率均高于Fe(VI)工艺。在最佳条件下([Fe(VI)]:[PMS] = 5:1,pH = 7.0,CPF = 2 mg / L),Fe(VI)/ PMS达到了94.75%的去除率,为82.80。仅Fe(VI)的%。提出了使用Fe(VI)/ PMS工艺的CPF降解途径,包括P = S键氧化,C-O键断裂,二乙基化以及羟基取代反应,从而导致生成六种产物。ECOSAR模拟程序显示,Fe(VI)/ PMS系统中CPF降解副产物的慢性和急性毒性相对降低。总而言之,研究表明,Fe(VI)/ PMS氧化是一种从水性介质中去除CPF的兼容且有效的技术。



























 京公网安备 11010802027423号
京公网安备 11010802027423号