Hydrometallurgy ( IF 4.7 ) Pub Date : 2021-04-18 , DOI: 10.1016/j.hydromet.2021.105607 Lei Zhang , Xue-yi Guo , Qing-hua Tian , Hong Qin
|
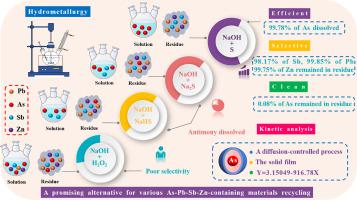
This work investigated the selective removal of arsenic from a lead‑antimony‑zinc-containing high arsenic dust in alkaline solution. The effects of sodium hydroxide (NaOH) concentration, temperature, leaching time, liquid-solid ratio (L/S) and the presence of elemental sulfur on the dissolution of arsenic (As), antimony (Sb), lead (Pb) and Zinc (Zn) in NaOH solution were studied. The results show that the addition of elemental sulfur can effectively restrain the leaching of lead, antimony and zinc from high arsenic dust. Antimony trioxide (Sb2O3), arsenic trioxide (As2O3), basic lead arsenate [Pb5(AsO4)3OH] and zinc arsenate [Zn3(AsO4)2] in the raw materials were converted into sodium antimonate hydrate [NaSb(OH)6], lead sulfide (PbS) and zinc sulfide (ZnS). Arsenic existed in the leaching solution in the form of AsO33− or AsO43−. 99.8% of Arsenic was leached under the optimized conditions, while about 98.2% of antimony, 99.9% of lead and 99.8% of zinc remained in the leach residue with the arsenic concentration less than 0.1%. In addition, the kinetics of arsenic leaching from high arsenic dust was investigated, with the calculated apparent activation energy of 7.62 kJ/mol. The results show that arsenic leaching is a diffusion-controlled process in the solid film, and the solid film was inferred as the lead sulfide, zinc sulfide and sodium antimonate hydrate. This study provides an efficient, clean and environmental-friendly method for arsenic separation from high arsenic dust, together with recovering valuable metals. It is expected to be a promising alternative for recycling various As-Pb-Sb-Zn-containing complicated materials.
中文翻译:

在NaOH-S系统中从高砷粉尘中选择性去除砷以及铅,锑,锌和锡的浸出行为
这项工作研究了在碱性溶液中从含铅锑锌的高砷粉尘中选择性去除砷的方法。氢氧化钠(NaOH)浓度,温度,浸出时间,液固比(L / S)和元素硫的存在对砷(As),锑(Sb),铅(Pb)和锌的溶解的影响研究了NaOH溶液中的(Zn)。结果表明,元素硫的添加可以有效地抑制高砷粉尘中铅,锑和锌的浸出。三氧化二锑(Sb 2 O 3),三氧化二砷(As 2 O 3),碱性砷酸铅[Pb 5(AsO 4)3 OH]和砷酸锌[Zn 3将原料中的(AsO 4)2 ]转化为锑酸钠水合物[NaSb(OH)6 ],硫化铅(PbS)和硫化锌(ZnS)。浸出溶液中的砷以AsO 3 3-或AsO 4 3-的形式存在。在优化条件下浸出99.8%的砷,浸出残渣中仍有约98.2%的锑,99.9%的铅和99.8%的锌残留,砷浓度小于0.1%。此外,还研究了从高砷粉尘中浸出砷的动力学,计算出的表观活化能为7.62 kJ / mol。结果表明,砷的浸出是固体膜中的扩散控制过程,推断固体膜为硫化铅,硫化锌和锑酸钠水合物。这项研究为从高砷粉尘中分离砷以及回收有价值的金属提供了一种高效,清洁和环保的方法。有望成为回收各种含As-Pb-Sb-Zn的复杂材料的有前途的替代方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号