当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Azo-hydrazone molecular switches: Synthesis and NMR conformational investigation
Magnetic Resonance in Chemistry ( IF 2 ) Pub Date : 2021-04-15 , DOI: 10.1002/mrc.5164 Atanas Kurutos 1 , Fadhil S Kamounah 2 , Georgi M Dobrikov 1 , Michael Pittelkow 2 , Stephan P A Sauer 2 , Poul Erik Hansen 3
Magnetic Resonance in Chemistry ( IF 2 ) Pub Date : 2021-04-15 , DOI: 10.1002/mrc.5164 Atanas Kurutos 1 , Fadhil S Kamounah 2 , Georgi M Dobrikov 1 , Michael Pittelkow 2 , Stephan P A Sauer 2 , Poul Erik Hansen 3
Affiliation
|
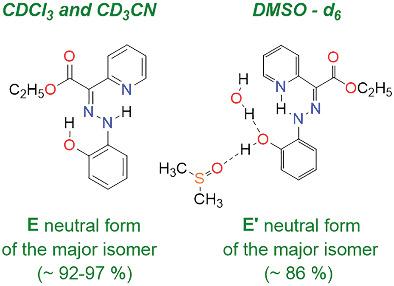
A series of five intramolecularly hydrogen-bonded arylhydrazone (aryl = phenol, p-nitrophenol, anisole, quinoline) derived molecular switches have been synthesized and characterized by NMR and HRMS techniques. It was found that the compounds exist as different isomers in solution. An investigation of both conformational and/or configurational changes of the azo-hydrazone compounds was carried out by 1D 1H- and 13C- spectra, 2D NOESY, COSY, HSQC, and HMBC techniques. It was found that these stimuli-responsive molecular switches exist mainly in the E form by intramolecularly hydrogen bonded between NH and the pyridine nitrogen at equilibrium. Deprotonation of the neutral E form yields the E′ deprotonated isomer. Prediction of 13C-NMR chemical shifts was achieved by DFT quantum mechanical calculations. Anions have traditionally been difficult to calculate correctly, so calculations of the anion using different functionals, basis sets, and solvent effects are also included. Deuterium isotope effects on the 13C-NMR chemical shifts were employed in the assignments and furthermore utilized as indicators of intramolecular hydrogen bonding. Studies in various organic solvents including CDCl3, CD3CN, and DMSO-d6 were also performed aiming to monitor dynamic changes over several days. The effect of the hydrogen bonded solvents leads to Z forms.
中文翻译:

偶氮腙分子开关:合成和核磁共振构象研究
一系列五个分子内氢键连接的芳基腙(芳基 = 苯酚、对硝基苯酚、苯甲醚、喹啉)衍生的分子开关已被合成并通过 NMR 和 HRMS 技术表征。发现这些化合物在溶液中以不同的异构体存在。通过1D 1 H-和13 C-光谱、2D NOESY、COSY、HSQC和HMBC技术对偶氮腙化合物的构象和/或构型变化进行了研究。发现这些刺激响应分子开关主要以 E 形式存在,通过分子内氢键连接在 NH 和平衡的吡啶氮之间。中性 E 形式的去质子化产生 E' 去质子化的异构体。预测13C-NMR 化学位移是通过 DFT 量子力学计算实现的。阴离子传统上难以正确计算,因此还包括使用不同泛函、基组和溶剂效应的阴离子计算。在分配中使用了氘同位素对13 C-NMR 化学位移的影响,并进一步用作分子内氢键的指示剂。还对包括CDCl 3、CD 3 CN 和DMSO-d 6在内的各种有机溶剂进行了研究,旨在监测几天内的动态变化。氢键溶剂的作用导致 Z 型。
更新日期:2021-04-15
中文翻译:

偶氮腙分子开关:合成和核磁共振构象研究
一系列五个分子内氢键连接的芳基腙(芳基 = 苯酚、对硝基苯酚、苯甲醚、喹啉)衍生的分子开关已被合成并通过 NMR 和 HRMS 技术表征。发现这些化合物在溶液中以不同的异构体存在。通过1D 1 H-和13 C-光谱、2D NOESY、COSY、HSQC和HMBC技术对偶氮腙化合物的构象和/或构型变化进行了研究。发现这些刺激响应分子开关主要以 E 形式存在,通过分子内氢键连接在 NH 和平衡的吡啶氮之间。中性 E 形式的去质子化产生 E' 去质子化的异构体。预测13C-NMR 化学位移是通过 DFT 量子力学计算实现的。阴离子传统上难以正确计算,因此还包括使用不同泛函、基组和溶剂效应的阴离子计算。在分配中使用了氘同位素对13 C-NMR 化学位移的影响,并进一步用作分子内氢键的指示剂。还对包括CDCl 3、CD 3 CN 和DMSO-d 6在内的各种有机溶剂进行了研究,旨在监测几天内的动态变化。氢键溶剂的作用导致 Z 型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号