当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Oxidation-assisted alkaline precipitation of nanoparticles using gas-diffusion electrodes
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2021-4-15 , DOI: 10.1039/d0re00463d Sam G. F. Eggermont 1, 2, 3, 4, 5 , Rafael Prato 3, 6, 7 , Xochitl Dominguez-Benetton 3, 4, 5, 6, 7 , Jan Fransaer 1, 2, 3
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2021-4-15 , DOI: 10.1039/d0re00463d Sam G. F. Eggermont 1, 2, 3, 4, 5 , Rafael Prato 3, 6, 7 , Xochitl Dominguez-Benetton 3, 4, 5, 6, 7 , Jan Fransaer 1, 2, 3
Affiliation
|
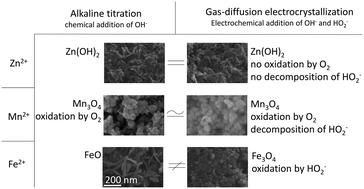
Metal oxide nanoparticles become increasingly important as functional materials because their diversity in composition and structure allow the control of their physical properties. This work investigates gas-diffusion electrocrystallization (GDEx) as a method to synthesize metal (oxy)(hydr)oxide nanoparticles (NPs) via oxidation-assisted alkaline precipitation (Ox-AP) by using gas-diffusion electrodes. GDEx was benchmarked against alkaline titration (AT). NPs were synthesized from ZnCl2, MnCl2, or FeCl2 precursor solutions at room temperature. Using AT, Zn(OH)2, Mn3O4, and FeO NPs were synthesized, respectively. Using GDEx, Zn(OH)2, Mn3O4, and Fe3O4 NPs were synthesized, respectively. The AT and GDEx process of the ZnCl2 and MnCl2 solutions demonstrated very similar pH behavior during precipitation and the Zn(OH)2 and Mn3O4 NPs synthesized with either technique were similar in size, morphology, and composition. For these cases, AT and GDEx both elicited alkaline precipitation and were considered equivalent processes for NP synthesis. In contrast, the AT and GDEx process of the FeCl2 solution demonstrated very different pH behavior during precipitation. Moreover, the FeO NPs, synthesized with AT, were much larger and of different shape and composition than the Fe3O4 NPs, synthesized with GDEx. The smaller sizes obtained with GDEx are suggested to result from an Ox-AP mechanism caused by the oxidation of Fe(II) to Fe(III) by H2O2 or HO2− during precipitation which presumably improves condensation kinetics and increases the supersaturation, both well-known size-determining factors.
中文翻译:

使用气体扩散电极的氧化辅助碱性沉淀纳米颗粒
金属氧化物纳米颗粒作为功能材料变得越来越重要,因为它们在组成和结构上的多样性可以控制其物理性质。这项工作研究了气体扩散电结晶(GDEx),作为一种使用气体扩散电极通过氧化辅助碱性沉淀(Ox-AP)合成金属(氧)(氢)氧化物纳米粒子(NPs )的方法。GDEx相对于碱滴定(AT)进行了基准测试。在室温下由ZnCl 2,MnCl 2或FeCl 2前驱体溶液合成NP 。使用AT分别合成了Zn(OH)2,Mn 3 O 4和FeO NPs。使用GDEx,Zn(OH)2分别合成了Mn 3 O 4和Fe 3 O 4 NP。ZnCl 2和MnCl 2溶液的AT和GDEx工艺在沉淀过程中显示出非常相似的pH行为,并且用任一种技术合成的Zn(OH)2和Mn 3 O 4 NP的尺寸,形态和组成均相似。对于这些情况,AT和GDEx都引起碱性沉淀,被认为是NP合成的等效过程。相比之下,FeCl 2的AT和GDEx工艺溶液在沉淀过程中表现出截然不同的pH行为。此外,与由GDEx合成的Fe 3 O 4 NPs相比,由AT合成的FeO NPs更大,形状和组成也不同。与GDEx获得的较小的尺寸建议从牛-AP机制造成的Fe(氧化导致II)与Fe(III由H)2 ö 2或HO 2 -沉淀过程中这大概提高缩合动力学并且增加了过饱和,这两个众所周知的大小决定因素。
更新日期:2021-04-15
中文翻译:

使用气体扩散电极的氧化辅助碱性沉淀纳米颗粒
金属氧化物纳米颗粒作为功能材料变得越来越重要,因为它们在组成和结构上的多样性可以控制其物理性质。这项工作研究了气体扩散电结晶(GDEx),作为一种使用气体扩散电极通过氧化辅助碱性沉淀(Ox-AP)合成金属(氧)(氢)氧化物纳米粒子(NPs )的方法。GDEx相对于碱滴定(AT)进行了基准测试。在室温下由ZnCl 2,MnCl 2或FeCl 2前驱体溶液合成NP 。使用AT分别合成了Zn(OH)2,Mn 3 O 4和FeO NPs。使用GDEx,Zn(OH)2分别合成了Mn 3 O 4和Fe 3 O 4 NP。ZnCl 2和MnCl 2溶液的AT和GDEx工艺在沉淀过程中显示出非常相似的pH行为,并且用任一种技术合成的Zn(OH)2和Mn 3 O 4 NP的尺寸,形态和组成均相似。对于这些情况,AT和GDEx都引起碱性沉淀,被认为是NP合成的等效过程。相比之下,FeCl 2的AT和GDEx工艺溶液在沉淀过程中表现出截然不同的pH行为。此外,与由GDEx合成的Fe 3 O 4 NPs相比,由AT合成的FeO NPs更大,形状和组成也不同。与GDEx获得的较小的尺寸建议从牛-AP机制造成的Fe(氧化导致II)与Fe(III由H)2 ö 2或HO 2 -沉淀过程中这大概提高缩合动力学并且增加了过饱和,这两个众所周知的大小决定因素。

























 京公网安备 11010802027423号
京公网安备 11010802027423号