当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
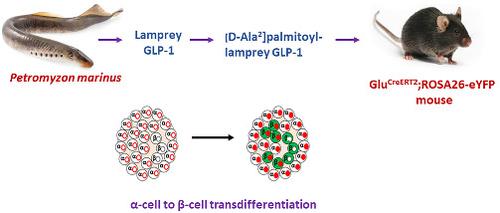
Effects of long-acting analogues of lamprey GLP-1 and paddlefish glucagon on alpha- to beta-cell transdifferentiation in an insulin-deficient transgenic mouse model
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2021-04-11 , DOI: 10.1002/psc.3328 Galyna V Graham 1 , J Michael Conlon 1 , R Charlotte Moffett 1 , Yasser H Abdel-Wahab 1 , Peter R Flatt 1
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2021-04-11 , DOI: 10.1002/psc.3328 Galyna V Graham 1 , J Michael Conlon 1 , R Charlotte Moffett 1 , Yasser H Abdel-Wahab 1 , Peter R Flatt 1
Affiliation
|
The abilities of the long-acting, dual-agonist anti-diabetic peptides [D-Ala2]palmitoyl-lamprey GLP-1 and [D-Ser2]palmitoyl-paddlefish glucagon to induce α-cell to β-cell transdifferentiation were investigated in GluCreERT2;ROSA26-eYFP mice. These animals have been genetically engineered so that yellow fluorescent protein is specifically expressed in glucagon-producing α-cells, thereby allowing cell lineage tracing. Insulin deficiency was produced by treatment of the mice with multiple low doses of streptozotocin. Administration of the peptides (twice daily intraperitoneal injections of 25 nmol/kg body weight over 10 days) to streptozotocin-treated mice produced significant (P < 0.05) increases in pancreatic insulin content and plasma insulin concentrations compared with control mice. Immunohistochemical studies demonstrated a significant (P < 0.05) increase in the % of cells staining for both insulin and fluorescent protein in islets located in the head region of the pancreas (from 10.0 ± 1.3% of total cells in untreated mice to 20.0 ± 3.85% in mice treated with D-Ala2]palmitoyl-lamprey GLP-1 and to 17.3 ± 1.1% in mice treated with [D-Ser2]palmitoyl-paddlefish glucagon). Corresponding effects upon islets in the tail region were not significant. The data indicate an improvement in β-cell mass and positive effects on transdifferentiation of glucagon-producing to insulin-producing cells. The study provides further evidence that proglucagon-derived peptides from phylogenetical ancient fish show therapeutic potential for treatment of diabetes.
中文翻译:

七鳃鳗 GLP-1 和白鲟胰高血糖素的长效类似物对胰岛素缺乏转基因小鼠模型中 α 到 β 细胞转分化的影响
研究了长效双激动剂抗糖尿病肽 [D-Ala 2 ] 棕榈酰-七鳃鳗 GLP-1 和 [D-Ser 2 ] 棕榈酰-白鲟胰高血糖素诱导 α 细胞向 β 细胞转分化的能力在 Glu CreERT2 中;ROSA26-eYFP 小鼠。这些动物经过基因工程改造,黄色荧光蛋白在产生胰高血糖素的 α 细胞中特异性表达,从而可以追踪细胞谱系。胰岛素缺乏是通过用多次低剂量链脲佐菌素治疗小鼠而产生的。对链脲佐菌素处理的小鼠施用肽(每天两次,每次腹膜内注射 25 nmol/kg 体重,持续 10 天)产生显着(P < 0.05) 与对照小鼠相比,胰腺胰岛素含量和血浆胰岛素浓度增加。免疫组织化学研究表明 ,位于胰腺头部区域的胰岛中胰岛素和荧光蛋白的细胞染色百分比显着增加 ( P < 0.05)(从未治疗小鼠总细胞的 10.0 ± 1.3% 增加到 20.0 ± 3.85%)在用 D-Ala 2治疗的小鼠中]棕榈酰七鳃鳗 GLP-1 在用 [D-Ser 2治疗的小鼠中达到 17.3 ± 1.1%]棕榈酰-白鲟胰高血糖素)。对尾部胰岛的相应影响不显着。数据表明 β 细胞质量有所改善,并对产生胰高血糖素的细胞向产生胰岛素的细胞的转分化产生积极影响。该研究提供了进一步的证据,表明来自系统发育古老鱼类的胰高血糖素原衍生肽显示出治疗糖尿病的治疗潜力。
更新日期:2021-04-11
中文翻译:

七鳃鳗 GLP-1 和白鲟胰高血糖素的长效类似物对胰岛素缺乏转基因小鼠模型中 α 到 β 细胞转分化的影响
研究了长效双激动剂抗糖尿病肽 [D-Ala 2 ] 棕榈酰-七鳃鳗 GLP-1 和 [D-Ser 2 ] 棕榈酰-白鲟胰高血糖素诱导 α 细胞向 β 细胞转分化的能力在 Glu CreERT2 中;ROSA26-eYFP 小鼠。这些动物经过基因工程改造,黄色荧光蛋白在产生胰高血糖素的 α 细胞中特异性表达,从而可以追踪细胞谱系。胰岛素缺乏是通过用多次低剂量链脲佐菌素治疗小鼠而产生的。对链脲佐菌素处理的小鼠施用肽(每天两次,每次腹膜内注射 25 nmol/kg 体重,持续 10 天)产生显着(P < 0.05) 与对照小鼠相比,胰腺胰岛素含量和血浆胰岛素浓度增加。免疫组织化学研究表明 ,位于胰腺头部区域的胰岛中胰岛素和荧光蛋白的细胞染色百分比显着增加 ( P < 0.05)(从未治疗小鼠总细胞的 10.0 ± 1.3% 增加到 20.0 ± 3.85%)在用 D-Ala 2治疗的小鼠中]棕榈酰七鳃鳗 GLP-1 在用 [D-Ser 2治疗的小鼠中达到 17.3 ± 1.1%]棕榈酰-白鲟胰高血糖素)。对尾部胰岛的相应影响不显着。数据表明 β 细胞质量有所改善,并对产生胰高血糖素的细胞向产生胰岛素的细胞的转分化产生积极影响。该研究提供了进一步的证据,表明来自系统发育古老鱼类的胰高血糖素原衍生肽显示出治疗糖尿病的治疗潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号