当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Thermodynamic Correlation of 4-(Hydroxymethyl) Benzoic Acid in Nine Pure Solvents and Two Binary Solvent Mixtures at (283.15–323.15) K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-04-08 , DOI: 10.1021/acs.jced.1c00035 Xiang Kang 1, 2 , Mengya Li 1, 2 , Jiahui Li 1, 2 , Kuo Wang 1, 2 , Dandan Han 1, 2 , Junbo Gong 1, 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-04-08 , DOI: 10.1021/acs.jced.1c00035 Xiang Kang 1, 2 , Mengya Li 1, 2 , Jiahui Li 1, 2 , Kuo Wang 1, 2 , Dandan Han 1, 2 , Junbo Gong 1, 2
Affiliation
|
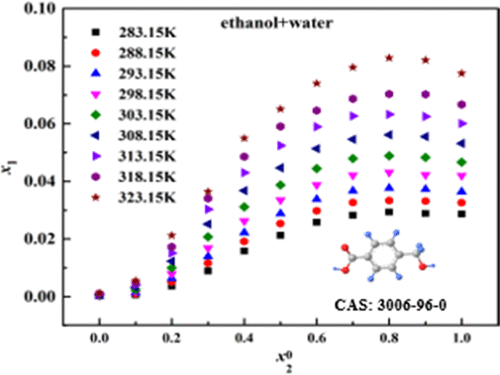
The solubility of 4-(hydroxymethyl) benzoic acid (p-HMBA) in nine pure solvents (water, methanol, ethanol, 1-propanol, 2-propanol, ethyl formate, ethyl acetate, isopropyl acetate, and butyl acetate) and two different binary solvents (methanol + water and ethanol + water) was measured by a gravimetric method at temperatures ranging from 283.15 to 323.15 K under atmospheric pressure (p = 0.1 MPa). The results reveal that the solubility of p-HMBA increases with increasing temperature in all investigated solvents. Under an isothermal condition, the solubility of p-HMBA increases monotonously with the increase of the mole fraction of methanol in binary solvents of methanol and water. However, the solubility of p-HMBA increases first and then decreases with the increase of ethanol in the binary solvent mixtures of ethanol and water and reaches its maximum value when the mole fraction of ethanol is 0.8. The solubility data were correlated with the modified Apelblat equation and λh model. All of the models gave satisfactory correlation results. The results presented in this paper would be helpful to the further crystallization and separation process of p-HMBA.
中文翻译:

4-(羟甲基)苯甲酸在(283.15–323.15)K下的九种纯溶剂和两种二元溶剂混合物中的溶解度测量和热力学相关性
4-(羟甲基)苯甲酸(p-HMBA)在九种纯溶剂(水,甲醇,乙醇,1-丙醇,2-丙醇,甲酸乙酯,乙酸乙酯,乙酸异丙酯和乙酸丁酯)中的溶解度在大气压力下,通过重量分析法在283.15至323.15 K的温度范围内测量二元溶剂(甲醇+水和乙醇+水)(p= 0.1 MPa)。结果表明,在所有研究的溶剂中,p-HMBA的溶解度均随温度的升高而增加。在等温条件下,p-HMBA的溶解度随着甲醇在甲醇和水的二元溶剂中的摩尔分数的增加而单调增加。然而,在乙醇和水的二元溶剂混合物中,p-HMBA的溶解度先增加,然后随乙醇的增加而降低,当乙醇的摩尔分数为0.8时,其溶解度达到最大值。溶解性数据均用改性Apelblat方程和λ相关ħ模型。所有模型都给出了令人满意的相关结果。本文提出的结果将有助于p-HMBA的进一步结晶和分离过程。
更新日期:2021-05-13
中文翻译:

4-(羟甲基)苯甲酸在(283.15–323.15)K下的九种纯溶剂和两种二元溶剂混合物中的溶解度测量和热力学相关性
4-(羟甲基)苯甲酸(p-HMBA)在九种纯溶剂(水,甲醇,乙醇,1-丙醇,2-丙醇,甲酸乙酯,乙酸乙酯,乙酸异丙酯和乙酸丁酯)中的溶解度在大气压力下,通过重量分析法在283.15至323.15 K的温度范围内测量二元溶剂(甲醇+水和乙醇+水)(p= 0.1 MPa)。结果表明,在所有研究的溶剂中,p-HMBA的溶解度均随温度的升高而增加。在等温条件下,p-HMBA的溶解度随着甲醇在甲醇和水的二元溶剂中的摩尔分数的增加而单调增加。然而,在乙醇和水的二元溶剂混合物中,p-HMBA的溶解度先增加,然后随乙醇的增加而降低,当乙醇的摩尔分数为0.8时,其溶解度达到最大值。溶解性数据均用改性Apelblat方程和λ相关ħ模型。所有模型都给出了令人满意的相关结果。本文提出的结果将有助于p-HMBA的进一步结晶和分离过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号