Journal of Structural Biology ( IF 3 ) Pub Date : 2021-04-02 , DOI: 10.1016/j.jsb.2021.107732 Nicolás González Bardeci 1 , Enzo Tofolón 1 , Felipe Trajtenberg 2 , Julio Caramelo 3 , Nicole Larrieux 2 , Silvia Rossi 1 , Alejandro Buschiazzo 2 , Silvia Moreno 1
|
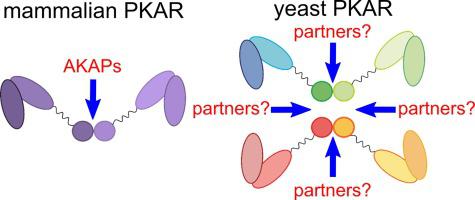
Protein Kinase A (PKA) is a widespread enzyme that plays a key role in many signaling pathways from lower eukaryotes to metazoans. In mammals, the regulatory (R) subunits sequester and target the catalytic (C) subunits to proper subcellular locations. This targeting is accomplished by the dimerization and docking (D/D) domain of the R subunits. The activation of the holoenzyme depends on the binding of the second messenger cAMP. The only available structures of the D/D domain proceed from mammalian sources. Unlike dimeric mammalian counterparts, the R subunit from Saccharomyces cerevisiae (Bcy1) forms tetramers in solution. Here we describe the first high-resolution structure of a non-mammalian D/D domain. The tetramer in the crystals of the Bcy1 D/D domain is a dimer of dimers that retain the classical D/D domain fold. By using phylogenetic and structural analyses combined with site-directed mutagenesis, we found that fungal R subunits present an insertion of a single amino acid at the D/D domain that shifts the position of a downstream, conserved arginine. This residue participates in intra-dimer interactions in mammalian D/D domains, while due to this insertion it is involved in inter-dimer contacts in Bcy1, which are crucial for the stability of the tetramer. This surprising finding challenges well-established concepts regarding the oligomeric state within the PKAR protein family and provides important insights into the yet unexplored structural diversity of the D/D domains and the molecular determinants of R subunit oligomerization.
中文翻译:

酵母调节亚基的晶体结构揭示了蛋白激酶 A 寡聚化的关键进化见解
蛋白激酶 A (PKA) 是一种广泛存在的酶,在从低等真核生物到后生动物的许多信号通路中起关键作用。在哺乳动物中,调节 (R) 亚基隔离并将催化 (C) 亚基定位到适当的亚细胞位置。这种靶向是通过 R 亚基的二聚化和对接 (D/D) 域来实现的。全酶的激活依赖于第二信使 cAMP 的结合。D/D 结构域的唯一可用结构来自哺乳动物来源。与二聚体哺乳动物对应物不同,来自酿酒酵母的 R 亚基(Bcy1) 在溶液中形成四聚体。在这里,我们描述了非哺乳动物 D/D 域的第一个高分辨率结构。Bcy1 D/D 结构域晶体中的四聚体是保留经典 D/D 结构域折叠的二聚体二聚体。通过使用系统发育和结构分析结合定点诱变,我们发现真菌 R 亚基在 D/D 结构域插入单个氨基酸,从而改变下游保守精氨酸的位置。该残基参与哺乳动物 D/D 结构域中的二聚体内相互作用,而由于这种插入,它参与了 Bcy1 中的二聚体间接触,这对四聚体的稳定性至关重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号