Protein Expression and Purification ( IF 1.6 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.pep.2021.105878 Carla Lizbeth Segovia-Trinidad 1 , Bastian Quaas 1 , Zhaopeng Li 1 , Antonina Lavrentieva 1 , Yvonne Roger 2 , Thomas Scheper 1 , Andrea Hoffmann 2 , Ursula Rinas 3
|
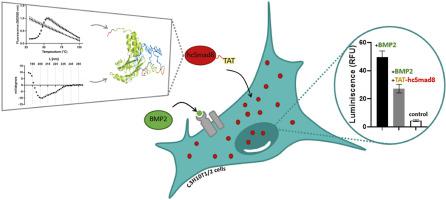
Smad8 is a transcriptional regulator that participates in the intracellular signaling pathway of the transforming growth factor-β (TGF-β) family. Full-length Smad8 is an inactive protein in the absence of ligand stimulation. The expression of a truncated version of the protein lacking the MH1 domain (cSmad8) revealed constitutive activity in genetically engineered mesenchymal stem cells and, in combination with BMP-2, exhibited a tendon cell-inducing potential. To further explore function and applicability of Smad8 in regenerative medicine recombinant production is required. Herein, we further engineered cSmad8 to include the transactivation signal (TAT) of the human immunodeficiency virus (HIV) to allow internalization into cells. TAT-hcSmad8 was produced in endotoxin-free ClearColi® BL21 (DE3), refolded from inclusion bodies (IBs) and purified by Heparin chromatography. Analysis of TAT-hcSmad8 by thermal shift assay revealed the formation of a hydrophobic core. The presence of mixed α-helixes and β-sheets, in line with theoretical models, was proven by circular dichroism. TAT-hcSmad8 was successfully internalized by C3H10T1/2 cells, where it was mainly found in the cytoplasm and partially in the nucleus. Finally, it was shown that TAT-hcSmad8 exhibited biological activity in C3H10T1/2 cells after co-stimulation with BMP-2.
中文翻译:

在 ClearColi® BL21 (DE3) 中作为包涵体产生的组成型活性人 Smad8 的重折叠、纯化和表征
Smad8 是一种转录调节因子,参与转化生长因子-β (TGF-β) 家族的细胞内信号通路。在没有配体刺激的情况下,全长 Smad8 是一种无活性的蛋白质。缺乏 MH1 结构域 (cSmad8) 的蛋白质截短版本的表达揭示了基因工程间充质干细胞的组成型活性,并与 BMP-2 结合,表现出诱导肌腱细胞的潜力。需要进一步探索 Smad8 在再生医学重组生产中的功能和适用性。在此,我们进一步设计了 cSmad8 以包含人类免疫缺陷病毒 (HIV) 的反式激活信号 (TAT),以允许内化到细胞中。TAT-hcSmad8 在无内毒素的 ClearColi® BL21 (DE3) 中产生,从包涵体 (IBs) 重新折叠并通过肝素层析纯化。通过热位移测定对 TAT-hcSmad8 的分析揭示了疏水核心的形成。圆二色性证明了与理论模型一致的混合α-螺旋和β-折叠的存在。TAT-hcSmad8 被 C3H10T1/2 细胞成功内化,主要存在于细胞质中,部分存在于细胞核中。最后,表明 TAT-hcSmad8 在与 BMP-2 共刺激后在 C3H10T1/2 细胞中表现出生物活性。它主要存在于细胞质中,部分存在于细胞核中。最后,表明 TAT-hcSmad8 在与 BMP-2 共刺激后在 C3H10T1/2 细胞中表现出生物活性。它主要存在于细胞质中,部分存在于细胞核中。最后,表明 TAT-hcSmad8 在与 BMP-2 共刺激后在 C3H10T1/2 细胞中表现出生物活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号