Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.jece.2021.105423 Wu Zhaozhao , Li Rongjuan , Pei Luyao , Yang yang , Li Xiaoguang , Jiang Juncen , Zhang Fan
|
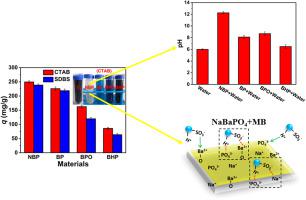
In this work, barium phosphate composites were easily modified by changing the reactants and surfactants to be barium sodium phosphate (NaBaPO4), barium phosphate (Ba3(PO4)2), barium hydroxyl phosphate (Ba5(PO4)3OH), and barium hydrogen phosphate (BaHPO4) (denoted NBP, BP, BPO, and BHP, respectively) using a one-step hydrothermal method. Positively charged cetyl trimethyl ammonium bromide (CTAB) on phosphate composites can slightly increase their removal capacities for methyl blue (MB) though it had no obvious effects on the composition and crystal type of materials. Dye removal experiments were carried out in a batch system to optimize operation variables such as solution pH, temperature, adsorbent dosage, contact time, and initial dye concentration. The kinetic behavior of all the materials agreed well with the pseudo-second-order model. The isothermal data of NBP and BPO fitted to the Langmuir model while Freundlich model was better to describe the isothermal data of BP and BHP. The removal capability for MB solution followed an order of NBP (249.6±6.0 mg/g) > BP (226.0±6.0 mg/g) > BPO (162.5±5.0 mg/g) > BHP (85.84±4.0 mg/g). NBP is most suitable for MB removal because of its high alkalinity: (1) The obvious increase of pH from 6.03±0.10 to 12.26±0.20 after adding NBP in solution can lead to improved decolorization of MB solution. (2) Strong Lewis acid base interaction between cations (Ba2+/Na+) in NBP and –SO3- in MB as well as the hydrogen bonding between PO43- in NBP and N–H or C–H in MB contributed to the high removal capacity of NBP.
中文翻译:

易于改性的磷酸钡复合材料,可从溶液中有效去除甲基蓝
在这项工作中,可以通过将反应物和表面活性剂更改为磷酸钡钠(NaBaPO 4),磷酸钡(Ba 3(PO 4)2),羟基磷酸钡(Ba 5(PO 4)3 OH )来轻松地改性磷酸钡复合材料。)和磷酸氢钡(BaHPO 4)(分别表示为NBP,BP,BPO和BHP)使用一步水热法。带正电荷的十六烷基三甲基溴化铵(CTAB)磷酸盐复合材料可稍微增加它们的去除能力FO ř甲基蓝(MB),尽管它对材料的组成和晶体类型没有明显影响。在间歇系统中进行染料去除实验,以优化操作变量,例如溶液的pH值,温度,吸附剂用量,接触时间和初始染料浓度。所有材料的动力学行为与拟二阶模型非常吻合。NBP和BPO的等温数据符合Langmuir模型,而Freundlich模型更适合描述BP和BHP的等温数据。MB溶液的去除能力依次为NBP(249.6±6.0 mg / g)> BP(226.0±6.0 mg / g)> BPO(162.5±5.0 mg / g)> BHP(85.84±4.0) 毫克/克)。NBP具有较高的碱度,因此最适合用于MB的去除:(1)在溶液中加入NBP后,pH值从6.03±0.10明显增加到12.26±0.20可以改善MB溶液的脱色。(2)强路易斯酸碱阳离子(巴之间的相互作用2+ /钠+在NBP)和-SO 3 -中MB以及PO之间的氢键4 3-中NBP和N-H或C-H在MB有助于提高NBP的去除能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号