Letters in Organic Chemistry ( IF 0.8 ) Pub Date : 2021-03-31 , DOI: 10.2174/1570178617999200602151152 Gumpula Prasoona 1 , Baireddy Kishore 1 , Gavaji Brahmeshwari 1
|
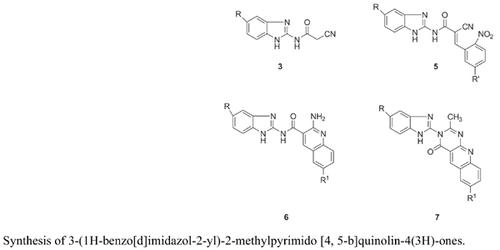
As infectious diseases causing bacteria and fungi are developing resistance to existing antimicrobial drugs, it is necessary to search for new drug targets with different structures and modes of action. Hence, it is essential to screen for new antimicrobial drugs with good efficacy and less toxicity. The reaction of 2-amino benzimidazoles 1 with ethyl cyanoacetate 2 afforded N-(1H-benzo[d]imidazol-2-yl)-2- cyanoacetamides 3. Compounds 3 on Knoevenagel condensation with o-nitro benzaldehydes 4 produced (E)-N-(1H-benzo[d]imidazol-2-yl)-2-cyano-3-(2-nitrophenol) acylamides 5. Compounds 5 were converted to 2-amino -N-(1H-benzo[d]imidazol-2-yl) quinoline-3-carboxamides 6 on treatment with stannous chloride by reductive cyclization. The target compounds viz., 3-(1H-benzo[d]imidazol-2-yl)-2- methylpyrimido [4, 5-b] quinolin-4(3H)-ones 7 were obtained by N-acetylation followed by cyclodehydration of compounds 6 in situ by treatment with acetic anhydride. 3-(1H-Benzo[d]imidazol-2-yl)-2- methylpyrimido [4, 5-b] quinolin-4(3H)-ones 7 have been synthesized from commercially available materials in excellent yields. The title compounds 7a-h are evaluated for in vitro antimicrobial activity. Compounds 7e, 7f and 7h have shown more antimicrobial activity than that of standard drugs. The structures of all the newly synthesized compounds 3, 5, 6 & 7 are confirmed on the basis of spectral data. Antimicrobial studies of compounds 7a-h have revealed that compounds 7e and 7f have more efficient activity when compared to the standard drugs.
中文翻译:

苯并咪唑基嘧啶[4,5-b]喹啉酮类化合物的合成及抗菌性能评价
由于引起细菌和真菌的传染病正在发展对现有抗菌药物的抗药性,因此有必要寻找具有不同结构和作用方式的新药物靶标。因此,筛选具有良好疗效和较低毒性的新型抗菌药物至关重要。2-氨基苯并咪唑1与氰基乙酸乙酯2反应,得到N-(1H-苯并[d]咪唑-2-基)-2-氰基乙酰胺3。化合物3在Knoevenagel上与邻硝基苯甲醛4缩合生成(E)- N-(1H-苯并[d]咪唑-2-基)-2-氰基-3-(2-硝基苯酚)酰基酰胺5。将化合物5转化为2-氨基-N-(1H-苯并[d]咪唑-用还原性环化作用用氯化亚锡处理2-基)喹啉3-甲酰胺6。目标化合物,即3-(1H-苯并[d]咪唑-2-基)-2-甲基嘧啶[4,通过N-乙酰化,然后通过用乙酸酐处理原位化合物6进行环脱水,获得5-b]喹啉-4(3H)-一。3-(1H-苯并[d]咪唑-2-基)-2-甲基嘧啶[4,5-b]喹啉-4(3H)-一7已由市售材料以优异的产率合成。评价标题化合物7a-h的体外抗菌活性。化合物7e,7f和7h具有比标准药物更高的抗菌活性。根据光谱数据确认所有新合成的化合物3、5、6和7的结构。化合物7a-h的抗菌研究表明,与标准药物相比,化合物7e和7f具有更高的活性。3-(1H-苯并[d]咪唑-2-基)-2-甲基嘧啶[4,5-b]喹啉-4(3H)-一7已由市售材料以优异的产率合成。评价标题化合物7a-h的体外抗菌活性。化合物7e,7f和7h具有比标准药物更高的抗菌活性。根据光谱数据确认所有新合成的化合物3、5、6和7的结构。化合物7a-h的抗菌研究表明,与标准药物相比,化合物7e和7f具有更高的活性。3-(1H-苯并[d]咪唑-2-基)-2-甲基嘧啶[4,5-b]喹啉-4(3H)-一7已由市售材料以优异的产率合成。评价标题化合物7a-h的体外抗菌活性。化合物7e,7f和7h具有比标准药物更高的抗菌活性。根据光谱数据确认所有新合成的化合物3、5、6和7的结构。化合物7a-h的抗菌研究表明,与标准药物相比,化合物7e和7f具有更高的活性。7f和7h显示出比标准药物更高的抗菌活性。根据光谱数据确认所有新合成的化合物3、5、6和7的结构。化合物7a-h的抗菌研究表明,与标准药物相比,化合物7e和7f具有更高的活性。7f和7h显示出比标准药物更高的抗菌活性。根据光谱数据确认所有新合成的化合物3、5、6和7的结构。化合物7a-h的抗菌研究表明,与标准药物相比,化合物7e和7f具有更高的活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号