Solid State Sciences ( IF 3.5 ) Pub Date : 2021-03-29 , DOI: 10.1016/j.solidstatesciences.2021.106603 O. Calixto-Lozada , J. Vazquez-Samperio , E. Córdoba-Tuta , E. Reguera , P. Acevedo-Peña
|
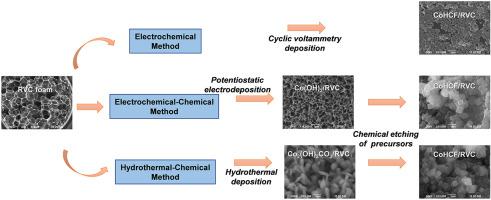
In the present work, particles of cobalt hexacyanoferrate (CoHCF) were deposited on reticulated vitreous carbon (RVC) foam surface to overcome the lack of conductivity of CoHCF and take advantage of their capacity for Na+ ions storage. The deposition was carried out through direct formation of the CoHCF by cyclic voltammetry, and the chemical transformation of cobalt hydroxide and cobalt hydroxide carbonate films obtained by electrochemical and hydrothermal synthesis, respectively, to CoHCF by immersion in an aqueous solution containing [Fe(CN)6]3- ions. The prepared composites were characterized through FTIR, Raman, XRD and SEM-EDS techniques to obtain information about their morphology, composition and structure. The results indicated that the employ of RVC foam leads to well-distributed CoHCF particles connected to its surface, offering abundant sites for ion-storage. Moreover, their electrochemical performance was evaluated by cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrochemical impedance spectroscopy (EIS). Benefiting from the highly conductive substrate, the charge storage mechanism of CoHCF and RVC, and the lower impedance originated at the CoHCF/RVC interface, the composites exhibited an enhanced performance at high potential scan rates or charge and discharge rates. Notably, improved electrochemical utilization of CoHCF was reached through the methods based on cobalt precursors, such composites displayed higher storage capacity. The CoHCF(-1.1 V)/RVC electrode synthesized by electrochemical-chemical synthesis exhibits a high specific capacity of 816 mC cm−3 at 0.25 mA cm−3 and 36% (296 mC cm−3) of its initial capacity was maintained at 5 mA cm−3, which improves 2.8 times the capacity achieved by CoHCF(50 mVs−1)/RVC through electrochemical strategy and the 19% of capacity retention from CoHCF(1 mmol)/RVC composite prepared by the hydrothermal-chemical method. Therefore, the CoHCF(-1.1 V)/RVC electrode can be potentially employed as a cathode in the recent sodium-ion batteries technology.
中文翻译:

通过在网状玻璃碳泡沫上进行电沉积和钴前体的化学刻蚀来生长六氰合铁酸钴颗粒,以用于Na离子电化学存储
在本工作中,六氰合铁酸钴(CoHCF)颗粒沉积在网状玻璃碳(RVC)泡沫表面上,以克服CoHCF的电导率不足,并利用其Na +离子存储能力。通过循环伏安法直接形成CoHCF,然后通过浸入含[Fe(CN)6 ] 3-离子。通过FTIR,拉曼,XRD和SEM-EDS技术对制备的复合材料进行表征,以获取有关其形态,组成和结构的信息。结果表明,使用RVC泡沫可导致分布均匀的CoHCF颗粒与其表面连接,从而为离子存储提供了丰富的位置。此外,通过循环伏安法(CV),恒电流充放电(GCD)和电化学阻抗谱(EIS)评估了它们的电化学性能。得益于高导电性基材,CoHCF和RVC的电荷存储机制以及源自CoHCF / RVC界面的较低阻抗,该复合材料在高电势扫描速率或充放电速率下表现出增强的性能。尤其,通过基于钴前体的方法可以提高CoHCF的电化学利用率,此类复合材料显示出更高的存储容量。电化学合成法制备的CoHCF(-1.1 V)/ RVC电极具有816 mC cm的高比容-3在0.25毫安厘米-3和36%(296三菱商事厘米-3其初始容量的)保持在5毫安厘米-3,这提高了2.8倍CoHCF达到的容量(50个MVS -1)/ RVC通过电化学水热化学法制备的CoHCF(1 mmol)/ RVC复合材料具有19%的容量保持率。因此,在最近的钠离子电池技术中,CoHCF(-1.1 V)/ RVC电极可以潜在地用作阴极。



























 京公网安备 11010802027423号
京公网安备 11010802027423号