Comparative Biochemistry and Physiology B: Biochemistry & Molecular Biology ( IF 2.2 ) Pub Date : 2021-03-27 , DOI: 10.1016/j.cbpb.2021.110598 Miho Miyakawa 1 , Tomohisa Katada 2 , Yunosuke Numa 1 , Tsutomu Kinoshita 1
|
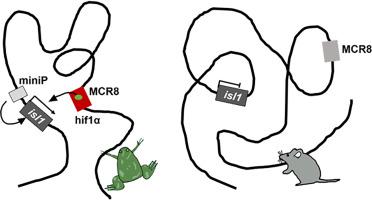
Adult mammalian hearts are not regenerative. However, recent studies have evidenced that hypoxia enhances their regeneration. Islet1 (isl1) is known as a cardiac progenitor marker, which is quiescent in adult mammal hearts. In Xenopus hearts, transcriptional activation of isl1 was shown during cardiac regeneration of froglets at 3 months after metamorphosis. In this study, we examined transcriptional regulation of isl1 focusing on hypoxia-inducible factor 1α (hif1α) in Xenopus heart. We found that hif1α expression was increased in response to cardiac injury and overexpression of hif1α upregulated mRNA expression of isl1. Multiple conservation analysis including 9 species revealed that 8 multiple conserved regions (MCRs) were present upstream of isl1. DNA sequence analysis using JASPAR showed hif1α binding motifs in MCRs. By luciferase reporter assay and chromatin immunoprecipitation analysis, we found that hif1α directly bound to hif1α motifs in the most distant MCR8 and showed a specific transcriptional activity on the MCR8. In the luciferase assay using constructs carrying MCR8 without a responsive motif of hif1α, the reporter activity was lost. Pharmacologically inhibition of hif1α affected isl1 transcription and downstream events including cardiac phenotypes, suggesting functional defects of islet1. Contrarily in murine hearts, transcription of isl1 was unresponsive even after cryoinjury to adult hearts while hif1α mRNA was induced. In comparative analysis of multiple alignment, hif1α elements present in MCR8 of Xenopus or zebrafish were found to be disrupted as species are evolutionarily distant from Xenopus and zebrafish. Our results suggested an altered switch of isl1 transcription between mammals and Xenopus laevis.
中文翻译:

非洲爪蟾islet1远端位点hif1α的转录调控元件
成年哺乳动物的心脏不能再生。然而,最近的研究表明,缺氧会促进它们的再生。Islet1 (isl1) 被称为心脏祖细胞标志物,在成年哺乳动物心脏中处于静止状态。在非洲爪蟾心脏中,在变态后 3 个月的小蛙心脏再生过程中显示了isl1 的转录激活。在这项研究中,我们检查了isl1 的转录调控,重点是非洲爪蟾心脏中的缺氧诱导因子 1α (hif1α) 。我们发现,HIF1α表达响应心脏损伤和HIF1α的表达增加上调的mRNA表达ISL1. 包括9个物种的多个保护分析表明,8个多发性保守的区域(MCRS)为上游存在ISL1。使用 JASPAR 的 DNA 序列分析显示 MCR 中存在 hif1α 结合基序。通过荧光素酶报告基因检测和染色质免疫沉淀分析,我们发现 hif1α 直接与最远的 MCR8 中的 hif1α 基序结合,并对 MCR8 显示出特异性转录活性。在使用携带 MCR8 的构建体而没有 hif1α 响应基序的荧光素酶测定中,报告基因活性丢失。hif1α 的药理学抑制影响isl1转录和下游事件,包括心脏表型,表明 islet1 的功能缺陷。相反,在小鼠心脏中,isl1 的转录即使在诱导 hif1α mRNA 的情况下对成人心脏进行冷冻损伤后,也没有反应。在多重比对的比较分析中,发现非洲爪蟾或斑马鱼的MCR8 中存在的 hif1α 元素被破坏,因为物种在进化上与非洲爪蟾和斑马鱼相距甚远。我们的结果表明哺乳动物和非洲爪蟾之间的isl1转录转换发生了改变。

























 京公网安备 11010802027423号
京公网安备 11010802027423号