Journal of Pharmaceutical Analysis ( IF 8.8 ) Pub Date : 2021-03-26 , DOI: 10.1016/j.jpha.2021.03.007 Katharina Rox 1, 2 , Silke Rath 3 , Dietmar H Pieper 3 , Marius Vital 3, 4 , Mark Brönstrup 1, 2
|
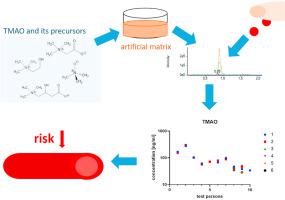
Trimethylamine-N-oxide (TMAO) has emerged as a potential biomarker for atherosclerosis and the development of cardiovascular diseases (CVDs). Although several clinical studies have shown striking associations of TMAO levels with atherosclerosis and CVDs, TMAO determinations are not clinical routine yet. The current methodology relies on isotope-labeled internal standards, which adds to pre-analytical complexity and costs for the quantification of TMAO and its precursors carnitine, betaine or choline. Here, we report a liquid chromatography-tandem mass spectrometry based method that is fast (throughput up to 240 samples/day), consumes low sample volumes (e.g., from a finger prick), and does not require isotope-labeled standards. We circumvented the analytical problem posed by the presence of endogenous TMAO and its precursors in human plasma by using an artificial plasma matrix for calibration. We cross-validated the results obtained using an artificial matrix with those using mouse plasma matrix and demonstrated that TMAO, carnitine, betaine and choline were accurately quantified in ‘real-life’ human plasma samples from healthy volunteers, obtained either from a finger prick or from venous puncture. Additionally, we assessed the stability of samples stored at −20 °C and room temperature. Whereas all metabolites were stable at −20 °C, increasing concentrations of choline were determined when stored at room temperature. Our method will facilitate the establishment of TMAO as a routine clinical biomarker in hematology in order to assess the risk for CVDs development, or to monitor disease progression and intervention effects.
中文翻译:

一种用于量化心血管疾病生物标志物三甲胺-N-氧化物及其前体的简化 LC-MS/MS 方法
三甲胺-N-氧化物 (TMAO) 已成为动脉粥样硬化和心血管疾病 (CVD) 发展的潜在生物标志物。尽管多项临床研究表明 TMAO 水平与动脉粥样硬化和 CVD 之间存在显着关联,但 TMAO 测定尚未成为临床常规。目前的方法依赖于同位素标记的内部标准,这增加了 TMAO 及其前体肉碱、甜菜碱或胆碱的定量分析前的复杂性和成本。在这里,我们报告了一种基于液相色谱-串联质谱的方法,该方法速度快(吞吐量高达 240 个样本/天),消耗的样本量小(例如,从手指刺伤),并且不需要同位素标记的标准。我们通过使用人工血浆基质进行校准,规避了人血浆中存在内源性 TMAO 及其前体所带来的分析问题。我们对使用人工基质获得的结果与使用小鼠血浆基质获得的结果进行了交叉验证,并证明 TMAO、肉碱、甜菜碱和胆碱在来自健康志愿者的“现实生活”人类血浆样本中被准确量化,这些样本来自手指刺痛或从静脉穿刺。此外,我们评估了储存在 -20 °C 和室温下的样品的稳定性。尽管所有代谢物在 -20 °C 下都是稳定的,但在室温下储存时,胆碱浓度会增加。我们的方法将有助于将 TMAO 确立为血液学中的常规临床生物标志物,以评估 CVD 发展的风险,


























 京公网安备 11010802027423号
京公网安备 11010802027423号