Journal of Geochemical Exploration ( IF 3.9 ) Pub Date : 2021-03-26 , DOI: 10.1016/j.gexplo.2021.106784 A. Sasmaz , P. Zuddas , M. Cangemi , D. Piazzese , G. Ozek , M. Venturi , P. Censi
|
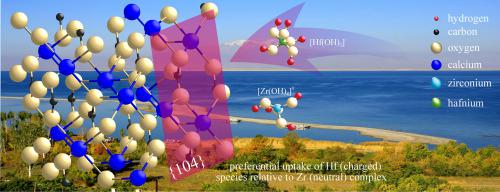
We investigated the distribution of Zr, Hf, and rare earth elements (REE) as the sum of lanthanides plus Y in the hydrothermal system in the Lake Van area of south-eastern Turkey. This system is characterised by water with variable pH in alkaline conditions resulting from hydrothermal CO2 upraise and neoformation of calcite minerals in near equilibrium with the interacting waters. Zr, Hf, and REE determinations were carried out for aqueous phases and suspended solids in lake water and surrounding thermal springs. We found that dissolved Hf is partitioned relative to Zr during calcite formation and that such fractionation is a function of the Ca2+ activity in warm water. The observed Zr Hf fractionation is explained by coulombic interactions that occur between suspended solid particles and dissolved phases at the calcite-water interface. There, the surfaces of carbonate minerals demonstrated greater reactivity towards aqueous Hf-bearing species relative to Zr-complexes. This evidence involves a coulombic mechanism of reactivity at the calcite-water interface because Hf complexes are negatively charged while Zr compounds are uncharged. Thus, authigenic calcite can behave as a suitable host for dissolved metal ion species to adsorb on crystal surfaces to remediate waste waters from mine drainage.
Hf fractionation is explained by coulombic interactions that occur between suspended solid particles and dissolved phases at the calcite-water interface. There, the surfaces of carbonate minerals demonstrated greater reactivity towards aqueous Hf-bearing species relative to Zr-complexes. This evidence involves a coulombic mechanism of reactivity at the calcite-water interface because Hf complexes are negatively charged while Zr compounds are uncharged. Thus, authigenic calcite can behave as a suitable host for dissolved metal ion species to adsorb on crystal surfaces to remediate waste waters from mine drainage.
中文翻译:

锆和water在中性-碱性水域中的分馏和稀土元素分布:土耳其范湖热液系统的案例研究
我们调查了土耳其东南部范湖地区热液系统中Zr,Hf和稀土元素(REE)的分布情况,这些元素是镧系元素和Y的总和。该系统的特点是在碱性条件下水的pH值可变,这是由热液CO 2升高和方解石矿物新形成而与相互作用的水接近平衡而产生的。对水相和湖水中及周围温泉中的悬浮固体进行了Zr,Hf和REE测定。我们发现,在方解石形成过程中,溶解的Hf相对于Zr是分配的,并且这种分馏是温水中Ca 2+活性的函数。观察到的Zr Hf分馏是通过在方解石-水界面处的悬浮固体颗粒和溶解相之间发生的库仑相互作用来解释的。那里,碳酸盐矿物的表面相对于Zr络合物对含Hf的水相具有更高的反应性。该证据涉及方解石-水界面的库仑反应性机制,因为H配合物带负电,而Zr化合物不带电。因此,自生方解石可以充当溶解的金属离子物质吸附在晶体表面上的适当基质,以补救矿山排水中的废水。
Hf分馏是通过在方解石-水界面处的悬浮固体颗粒和溶解相之间发生的库仑相互作用来解释的。那里,碳酸盐矿物的表面相对于Zr络合物对含Hf的水相具有更高的反应性。该证据涉及方解石-水界面的库仑反应性机制,因为H配合物带负电,而Zr化合物不带电。因此,自生方解石可以充当溶解的金属离子物质吸附在晶体表面上的适当基质,以补救矿山排水中的废水。


























 京公网安备 11010802027423号
京公网安备 11010802027423号