Journal of Water Process Engineering ( IF 7 ) Pub Date : 2021-03-25 , DOI: 10.1016/j.jwpe.2021.102027 Tomohito Kameda , Kazuya Horikoshi , Fumihiko Kitagawa , Shogo Kumagai , Yuko Saito , Masayuki Kondo , Yoichi Jimbo , Toshiaki Yoshioka
|
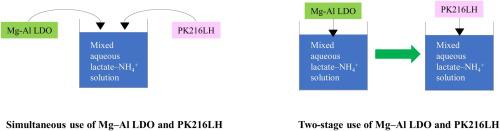
The proliferation of pluripotent stem cells in mass cell culture is hindered by the release of cell metabolites such as lactate and ammonia. In this work, a porous strong-acid cation exchange resin (PK216LH) and Mg–Al layered doubled oxide (LDO) were used to take up NH4+ and lactate, respectively. Initially, PK216LH could take up NH4+ from an aqueous solution. The uptake of NH4+ by PK216LH followed pseudo-second-order kinetics and corresponded to the Langmuir adsorption isotherm, with an apparent activation energy of 28.2 kJ/mol and a maximum adsorption amount of 1.38 mmol/g. The simultaneous use of Mg–Al LDO and PK216LH could not sufficiently take up lactate and NH4+. In contrast, the individual use of Mg–Al LDO and PK216LH in two stages enabled efficient removal of lactate and NH4+.
中文翻译:

阳离子交换树脂吸收氨的动力学和吸附等温线以及Mg-Al层状双氧化物和树脂处理混合的乳酸盐-氨水
多能干细胞在大规模细胞培养中的增殖受到诸如乳酸和氨等细胞代谢产物的释放的阻碍。在这项工作中,使用多孔强酸阳离子交换树脂(PK216LH)和Mg-Al层状双氧化物(LDO)分别吸收NH 4 +和乳酸。最初,PK216LH可以从水溶液中吸收NH 4 +。PK216LH吸收NH 4 +的过程遵循拟二级动力学,对应于Langmuir吸附等温线,表观活化能为28.2 kJ / mol,最大吸附量为1.38 mmol / g。同时使用Mg-Al LDO和PK216LH不能充分吸收乳酸和NH 4 +。相反,在两个阶段中单独使用Mg-Al LDO和PK216LH可以有效去除乳酸和NH 4 +。



























 京公网安备 11010802027423号
京公网安备 11010802027423号