Cell Calcium ( IF 4 ) Pub Date : 2021-03-17 , DOI: 10.1016/j.ceca.2021.102391 Sebastian Pantke 1 , Tabea C Fricke 1 , Mirjam J Eberhardt 1 , Christine Herzog 1 , Andreas Leffler 1
|
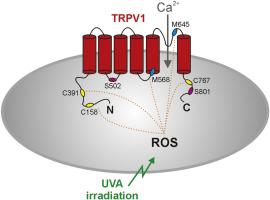
Redox-sensitivity is a common property of several transient receptor potential (TRP) ion channels. Oxidants and UVA-light activate TRPV2 by oxidizing methionine pore residues which are conserved in the capsaicin-receptor TRPV1. However, the redox-sensitivity of TRPV1 is regarded to depend on intracellular cysteine residues. In this study we examined if TRPV1 is gated by UVA-light, and if the conserved methionine residues are relevant for redox-sensitivity of TRPV1. Patch clamp recordings were performed to explore wildtype (WT) and mutants of human TRPV1 (hTRPV1).
UVA-light induced hTRPV1-mediated membrane currents and potentiated both proton- and heat-evoked currents. The reducing agent dithiothreitol (DTT) prevented and partially reversed UVA-light induced sensitization of hTRPV1. UVA-light induced sensitization was reduced in the mutant hTRPV1-C158A/C387S/C767S (hTRPV1-3C). The remaining sensitivity to UVA-light of hTRRPV1-3C was not further reduced upon exchange of the methionine residues M568 and M645. While UVA-induced sensitization was reduced in the protein kinase C-insensitive mutant hTRPV1-S502A/S801A, the PKC-inhibitors chelerythrine chloride, staurosporine and Gö6976 did not reduce UVA-induced effects on hTRPV1-WT. While hTRPV1-3C was insensitive to the cysteine-selective oxidant diamide, it displayed a residual sensitivity to H2O2 and chloramine-T. However, the exchange of M568 and M645 in hTRPV1-3C did not further reduce these effects.
Our data demonstrate that oxidants and UVA-light gate hTRPV1 by cysteine-dependent as well as cysteine-independent mechanisms. In contrast to TRPV2, the methionine residues 568 and 645 seem to be of limited relevance for redox-sensitivity of hTRPV1. Finally, UVA-light induced gating of hTRPV1 does not seem to require activation of protein kinase C.
中文翻译:

UVA 光和氧化剂对辣椒素受体 TRPV1 的门控由不同的机制介导
氧化还原敏感性是几种瞬时受体电位 (TRP) 离子通道的共同特性。氧化剂和 UVA 光通过氧化在辣椒素受体 TRPV1 中保守的蛋氨酸孔残基来激活 TRPV2。然而,TRPV1 的氧化还原敏感性被认为取决于细胞内的半胱氨酸残基。在这项研究中,我们检查了 TRPV1 是否被 UVA 光门控,以及保守的蛋氨酸残基是否与 TRPV1 的氧化还原敏感性相关。进行膜片钳记录以探索人类 TRPV1 (hTRPV1) 的野生型 (WT) 和突变体。
UVA 光诱导 hTRPV1 介导的膜电流并增强质子和热诱发电流。还原剂二硫苏糖醇 (DTT) 可防止并部分逆转 UVA 光诱导的 hTRPV1 致敏。在突变体 hTRPV1-C158A/C387S/C767S (hTRPV1-3C) 中,UVA 光诱导的致敏作用降低。在交换甲硫氨酸残基 M568 和 M645 后,hTRRPV1-3C 对 UVA 光的剩余敏感性没有进一步降低。虽然在蛋白激酶 C 不敏感突变体 hTRPV1-S502A/S801A 中 UVA 诱导的致敏作用降低,但 PKC 抑制剂氯化白屈菜红素、星形孢菌素和 Gö6976 并未降低 UVA 诱导对 hTRPV1-WT 的影响。虽然 hTRPV1-3C 对半胱氨酸选择性氧化二酰胺不敏感,但它对 H 2 O 2表现出残留敏感性和氯胺-T。然而,hTRPV1-3C 中 M568 和 M645 的交换并没有进一步降低这些影响。
我们的数据表明,氧化剂和 UVA 光通过半胱氨酸依赖性和半胱氨酸非依赖性机制来控制 hTRPV1。与 TRPV2 相比,蛋氨酸残基 568 和 645 似乎与 hTRPV1 的氧化还原敏感性相关性有限。最后,UVA 光诱导 hTRPV1 的门控似乎不需要激活蛋白激酶 C。


























 京公网安备 11010802027423号
京公网安备 11010802027423号