Journal of Industrial and Engineering Chemistry ( IF 6.1 ) Pub Date : 2021-03-17 , DOI: 10.1016/j.jiec.2021.03.019 Mirhossein Taheriotaghsara , Maria Bonto , Hamid M. Nick , Ali Akbar Eftekhari
|
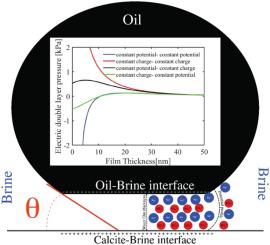
The shift in the wetting conditions during injection of modified salinity water (MSW) in carbonate reservoirs has been interpreted in several recent works through the DLVO extended theory. Two simplifications are usually adopted when applying the DLVO extended theory: (i) the electrostatic energy interaction is quantified by an analytical solution developed for systems containing only monovalent ions and (ii) the structural forces are independent of the type of brine. We address those by prioritizing the brine chemistry. We initially calculate the potential at the mineral and oil surfaces using two different surface complexation models implemented in Phreeqc and then we quantify the electrostatic forces by solving numerically the Poisson Boltzmann (PB) equations for non/symmetrical electrolytes. We observe that not only the identity of the ions, but also, more importantly, the boundary conditions (constant surface charge or constant surface potential) considered for the solution of PB can drastically modify the calculated electrostatic energy profile. We then calculate the total interaction energy and estimate a microscopic contact angle that is consistent with measured values. Our calculations show that increasing the concentration of salts such as MgCl2, CaCl2, and MgSO4 leads to more water-wet conditions, whereas salts like NaCl, KCl, and Na2SO4 show the opposite effect.
中文翻译:

使用表面力估算方解石的可湿性
通过DLVO扩展理论,在最近的几篇著作中已经解释了在碳酸盐岩储层中注入改良盐度水(MSW)期间润湿条件的变化。在应用DLVO扩展理论时,通常采用两种简化方法:(i)通过为仅包含一价离子的系统开发的分析解决方案来量化静电能相互作用,并且(ii)结构力与盐水的类型无关。我们通过优先考虑盐水化学来解决这些问题。我们首先使用在Phreeqc中实现的两种不同的表面络合模型来计算矿物和石油表面的电势,然后我们通过数值求解非/对称电解质的泊松玻耳兹曼(PB)方程来量化静电力。我们观察到,不仅离子的身份,而且,更重要的是,考虑到PB溶液的边界条件(恒定的表面电荷或恒定的表面电势)可以极大地改变计算出的静电能分布。然后,我们计算总的相互作用能并估计与测量值一致的微观接触角。我们的计算表明,增加诸如MgCl等盐的浓度2,CaCl 2和MgSO 4导致更多的水湿条件,而盐如NaCl,KCl和Na 2 SO 4显示相反的效果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号