Gondwana Research ( IF 6.1 ) Pub Date : 2021-03-13 , DOI: 10.1016/j.gr.2021.02.019 Altyna Bekhtenova , Anton Shatskiy , Ivan V. Podborodnikov , Anton V. Arefiev , Konstantin D. Litasov
|
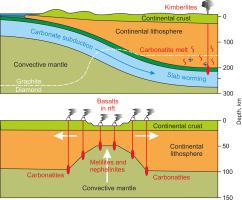
Phase relations in the carbonate constituent of mafic and ultramafic suites are decisive in the deep carbon cycle and partial melting in the Earth's mantle. Here we present an experimental study of phase relations in the “Na‑carbonatite” and “K‑carbonatite” systems, which represent the carbonate constituent of mantle eclogites and peridotites, respectively.
The phase relations in the “Na‑carbonatite” system indicate that the carbonate constituent of the mafic suite consists of Na2Ca4(CO3)5 + dolomite + eitelite, Na2Mg(CO3)2, at shallow mantle pressures and changes to Na2Ca4(CO3)5 + magnesite at depths exceeding 170 km. At the same time, the “K‑carbonatite” system indicates that the carbonate constituent of the ultramafic suite consists of K2Mg(CO3)2 + dolomite + magnesite at shallow mantle pressures and changes to K2Mg(CO3)2 + magnesite + aragonite at depths exceeding 170–180 km.
The “Na‑carbonatite” solidus is located near 850 °C / 3 GPa and 1100 °C / 6.5 GPa and has a Clapeyron slope of 14 MPa/°C. The “K‑carbonatite” solidus is situated near 825 °C / 3 GPa and 1000 °C / 6.5 GPa and has a Clapeyron slope of 20 MPa/°C. Both solidi are hotter than subduction geotherms. Consequently, under anhydrous conditions, the carbonate constituent of mafic and ultramafic suites can survive subduction into the deep mantle without melting. However, warming even to moderate temperatures corresponding to continental geotherms should be accompanied by partial melting yielding alkaline carbonatite melt.
中文翻译:

俯冲和大陆地热沿碳酸化榴辉岩和橄榄岩碳酸盐组分的相关系
镁铁质和超镁铁质套件中碳酸盐成分的相关系对深层碳循环和地幔的部分融化起决定性作用。在此,我们对“钠碳酸盐”和“ K碳酸盐”系统中的相关系进行实验研究,它们分别表示地幔榴辉岩和橄榄岩的碳酸盐成分。
“ Na-碳酸盐岩”系统中的相关系表明,镁铁矿组的碳酸盐成分由Na 2 Ca 4(CO 3)5 +白云石+沸石,Na 2 Mg(CO 3)2,地幔压力和 在深度超过170 km时变为Na 2 Ca 4(CO 3)5 +菱镁矿。同时,“钾碳酸盐”系统表明,超镁铁质组中的碳酸盐成分由 地幔压力较浅的K 2 Mg(CO 3)2 +白云石+菱镁矿组成,并转变为K 2。Mg(CO 3)2 +菱镁矿+文石的深度超过170-180 km。
“碳酸钠盐”固相线位于850°C / 3 GPa和1100°C / 6.5 GPa附近,并且Clapeyron斜率为14 MPa /°C。“ K-碳酸盐岩”固相线位于825°C / 3 GPa和1000°C / 6.5 GPa附近,并且Clapeyron斜率为20 MPa /°C。两种固体都比俯冲地热更热。因此,在无水条件下,镁铁质和超镁铁质套件的碳酸盐成分可以在不融化的情况下潜入深层地幔中。但是,即使变暖到与大陆地热相对应的中等温度,也应伴随部分熔化,从而生成碱性碳酸盐岩熔体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号