Journal of Molecular Graphics and Modelling ( IF 2.9 ) Pub Date : 2021-03-12 , DOI: 10.1016/j.jmgm.2021.107898 Qifei Wang 1 , Fei Chen 2 , Peng Liu 3 , Yushu Mu 1 , Shibin Sun 1 , Xulong Yuan 1 , Pan Shang 1 , Bo Ji 1
|
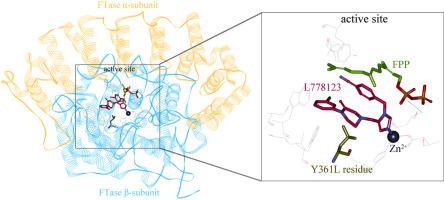
Oncogenic protein farnesyltransferase (FTase) is a key enzyme responsible for the lipid modification of a large and important number of proteins including Ras, which has been recognized as a druggable target of diverse cancers. Here, we report a systematic scaffold-based analysis to investigate the affinity, selectivity and cross-reactivity of nonpeptide inhibitors across ontology-enriched, disease-associated FTase mutants, by integrating multiple similarity matching, binding affinity scoring and enzyme inhibition assay. It is revealed that nonpeptide inhibitors are generally insensitive to FTase mutations; many of them cannot definitely select for wild-type target over mutant enzymes. Therefore, off-target is observed as a common phenomenon for the untargeted consequence of targeted therapies with FTase inhibition. This is not unexpected if considering that the enzyme active site is highly conserved in composition, configuration and function. The off-target, on the one hand, causes nonpeptide inhibitors with adverse drug reactions and, on the other hand, makes the inhibitors as promising candidates for the new use of old drugs. To practice the latter, a number of unexpected mutant–inhibitor interactions involved in cancer signaling pathways are uncovered in the created profile, from which several nonpeptide inhibitors are identified as insensitive to a drug-resistant mutation. Structural analysis suggests that the inhibitor ligands can bind to the mutant active site in a similar manner with wild-type target, although their nonbonded interactions appear to be impaired moderately upon the mutation.
中文翻译:

基于支架的非肽致癌性FTase抑制剂分析,采用多重相似性匹配,结合亲和力评分和酶抑制试验
致癌蛋白法尼基转移酶(FTase)是负责对包括Ras在内的大量重要蛋白质进行脂质修饰的关键酶,Ras被认为是多种癌症的可治疗靶标。在这里,我们报告了一个系统的基于支架的分析,以通过整合多个相似性匹配,结合亲和力得分和酶抑制试验,研究非肽类抑制剂跨本体富集的,疾病相关的FTase突变体的亲和力,选择性和交叉反应性。揭示了非肽抑制剂通常对FTase突变不敏感。他们中的许多人绝对不能选择突变型酶以外的野生型靶标。因此,对于具有FTase抑制作用的靶向治疗的未靶向结果,脱靶被视为一种常见现象。如果考虑到酶活性位点在组成,构型和功能上高度保守,这并不意外。脱靶一方面导致具有不良药物反应的非肽抑制剂,另一方面使抑制剂成为旧药物新用途的有希望的候选者。为了实践后者,在创建的图谱中发现了许多与癌症信号通路有关的意外突变体-抑制剂相互作用,从中可以鉴定出几种非肽类抑制剂对耐药性突变不敏感。结构分析表明,抑制剂配体可以以与野生型靶标相似的方式结合至突变活性位点,尽管它们的非键合相互作用似乎在突变后受到了一定程度的损害。



























 京公网安备 11010802027423号
京公网安备 11010802027423号