当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quaternary System Thermodynamic Investigation in a Mixed Solvent: NaCl + KCl + N,N-Dimethylformamide + H2O
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-03-10 , DOI: 10.1021/acs.jced.0c00899 Vahideh Masumi 1 , Rahman Salamat-Ahangari 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-03-10 , DOI: 10.1021/acs.jced.0c00899 Vahideh Masumi 1 , Rahman Salamat-Ahangari 1
Affiliation
|
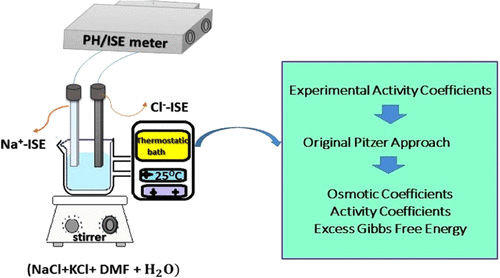
This article focuses on measuring and modeling activity coefficients and some thermodynamic properties of the quaternary system (NaCl + KCl + N,N-dimethylformamide (DMF) + H2O) using a potentiometric technique at (298.15 ± 0.05) K. By employing ion-selective electrodes (Na+-ISE, Cl–-ISE), experimental results were collated for the four series of the mixed electrolyte systems in various ionic strengths for the investigated quaternary system. The electrochemical measurements were carried out over total ionic strengths range from 0.0249 up to about 3.8223 mol·kg–1 in mixed N,N-dimethylformamide + H2O solvents, with various solvent mass percents (wt % = 0.0, 0.10, 0.20, and 0.30) at 298.15 ± 0.05 K. Experimental data were modeled on the basis of the Pitzer approach, and the two and three particles mixing ionic interaction parameters (θNaK and ψNaKCl) estimated for the quaternary electrolyte systems under investigation. Finally, using the optimized parameters (θNaK and ψNaKCl), the thermodynamic properties of electrolyte systems, such as activity coefficients of the constituent’s electrolyte, osmotic coefficients, and the excess Gibbs free energy, were determined for each mass percent of DMF in water for mixtures of sodium and potassium salts with common anion chloride. The resultant activity coefficients demonstrate a decreasing trend upon decreasing dielectric constants and as the mixed solvent medium growing dense and viscous.
中文翻译:

混合溶剂中的四元体系热力学研究:NaCl + KCl + N,N-二甲基甲酰胺+ H 2 O
本文着重于使用(298.15±0.05)K的电位计,对四元体系(NaCl + KCl + N,N-二甲基甲酰胺(DMF)+ H 2 O)的活度系数和一些热力学性质进行测量和建模。 -选择电极(Na + -ISE,Cl -- ISE),整理了四个系列的混合电解质系统在不同离子强度下用于研究的四元体系的实验结果。在混合的N,N-二甲基甲酰胺+ H 2中,在总离子强度范围为0.0249至约3.8223 mol·kg –1的条件下进行了电化学测量O型溶剂,在298.15±0.05 K时具有各种溶剂质量百分比(wt%= 0.0、0.10、0.20和0.30)。基于Pitzer方法以及将两个和三个粒子混合了离子相互作用参数( θ的NaK和ψ NaKCl)估计为所研究的季电解质系统。最后,使用优化的参数(θ的NaK和ψ NaKCl),对于钠盐和钾盐与普通阴离子氯化物的混合物,对于水中DMF的每个质量百分比,确定了电解质系统的热力学性质,例如该成分的电解质的活度系数,渗透系数和过量的吉布斯自由能。所得的活度系数显示出随着介电常数的降低以及混合溶剂介质的致密和粘稠度的增加而降低的趋势。
更新日期:2021-04-08
中文翻译:

混合溶剂中的四元体系热力学研究:NaCl + KCl + N,N-二甲基甲酰胺+ H 2 O
本文着重于使用(298.15±0.05)K的电位计,对四元体系(NaCl + KCl + N,N-二甲基甲酰胺(DMF)+ H 2 O)的活度系数和一些热力学性质进行测量和建模。 -选择电极(Na + -ISE,Cl -- ISE),整理了四个系列的混合电解质系统在不同离子强度下用于研究的四元体系的实验结果。在混合的N,N-二甲基甲酰胺+ H 2中,在总离子强度范围为0.0249至约3.8223 mol·kg –1的条件下进行了电化学测量O型溶剂,在298.15±0.05 K时具有各种溶剂质量百分比(wt%= 0.0、0.10、0.20和0.30)。基于Pitzer方法以及将两个和三个粒子混合了离子相互作用参数( θ的NaK和ψ NaKCl)估计为所研究的季电解质系统。最后,使用优化的参数(θ的NaK和ψ NaKCl),对于钠盐和钾盐与普通阴离子氯化物的混合物,对于水中DMF的每个质量百分比,确定了电解质系统的热力学性质,例如该成分的电解质的活度系数,渗透系数和过量的吉布斯自由能。所得的活度系数显示出随着介电常数的降低以及混合溶剂介质的致密和粘稠度的增加而降低的趋势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号