当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structure refinements of stoichiometric Ni3Se2 and NiSe
Acta Crystallographica Section C ( IF 0.8 ) Pub Date : 2021-03-09 , DOI: 10.1107/s2053229621002187 Kohei Unoki , Akira Yoshiasa , Ginga Kitahara , Tadao Nishiayama , Makoto Tokuda , Kazumasa Sugiyama , Akihiko Nakatsuka
Acta Crystallographica Section C ( IF 0.8 ) Pub Date : 2021-03-09 , DOI: 10.1107/s2053229621002187 Kohei Unoki , Akira Yoshiasa , Ginga Kitahara , Tadao Nishiayama , Makoto Tokuda , Kazumasa Sugiyama , Akihiko Nakatsuka
|
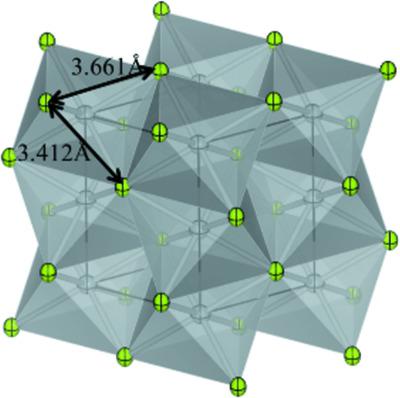
Single crystals of Ni3Se2 (trinickel diselenide) and NiSe (nickel selenide) with stoichiometric chemical compositions were grown in evacuated silica‐glass tubes. The chemical compositions of the single crystals of Ni3Se2 and NiSe were determined by scanning electron microscopy and energy‐dispersive X‐ray spectroscopy (SEM/EDS). The crystal structures of Ni3Se2 [rhombohedral, space group R32, a = 6.02813 (13), c = 7.24883 (16) Å, Z = 3] and NiSe [hexagonal, space group P63/mmc, a = 3.66147 (10), c = 5.35766 (16) Å, Z = 2] were analyzed by single‐crystal X‐ray diffraction and refined to yield R values of 0.020 and 0.018 for 117 and 85 unique reflections, respectively, with Fo > 4σ(Fo). R32 is a Sohncke type of space group where enantiomeric structures can exist; the single‐domain structure obtained by the refinement was confirmed to be correct by a Flack parameter of −0.05 (2). The existence of Ni—Ni bonds was confirmed in both compounds, in addition to the Ni—Se bonds. The value of the atomic displacement parameter (mean‐square displacement) of each atom in NiSe was larger than that in Ni3Se2. The larger amplitude of the atoms in NiSe corresponds to longer Ni—Se and Ni—Ni bond lengths in NiSe than in Ni3Se2. The Debye temperatures, gθD, estimated from observed mean‐square displacements for Ni and Se in Ni3Se2, were 322 and 298 K, respectively, while those for Ni and Se in NiSe were 246 and 241 K, respectively. The existence of large cavities in the structure and the weak bonding force are likely responsible for the brittle and soft nature of the NiSe crystal.
中文翻译:

化学计量的Ni3Se2和NiSe的晶体结构细化
在真空石英玻璃管中生长具有化学计量化学成分的Ni 3 Se 2(三硒二镍)和NiSe(硒化镍)单晶。Ni 3 Se 2和NiSe单晶的化学组成通过扫描电子显微镜和能量色散X射线光谱法(SEM / EDS)确定。Ni 3 Se 2 [菱形,空间群R 32,a = 6.02813(13),c = 7.24883(16)Å,Z = 3]和NiSe [六角形,空间群P 6 3 / mmc,a的晶体结构 = 3.66147(10),c = 5.35766(16)Å,Z = 2]通过单晶X射线衍射分析并进行精制,分别得到117和85个唯一反射的R值分别为0.020和0.018,其中F o >4σ(F o)。R 32是其中存在对映体结构的Sohncke型空间群;通过-0.05(2)的Flack参数确认了通过细化获得的单域结构是正确的。除Ni-Se键外,在两种化合物中均证实了Ni-Ni键的存在。NiSe中每个原子的原子位移参数(均方位移)值大于Ni 3 Se 2中的原子位移参数(均方位移)值。NiSe中原子的更大振幅对应于NiSe中比Ni 3 Se 2中更长的Ni-Se和Ni-Ni键长。德拜温度,Gθ d,从观察到的均方位移为Ni和硒中的Ni估计3硒2,分别为322和298 K,分别,而对于Ni和硒NISE分别为246和241 K,分别。NiSe晶体的脆性和柔软性可能是由于结构中存在大的空穴以及弱的结合力所致。
更新日期:2021-04-05
中文翻译:

化学计量的Ni3Se2和NiSe的晶体结构细化
在真空石英玻璃管中生长具有化学计量化学成分的Ni 3 Se 2(三硒二镍)和NiSe(硒化镍)单晶。Ni 3 Se 2和NiSe单晶的化学组成通过扫描电子显微镜和能量色散X射线光谱法(SEM / EDS)确定。Ni 3 Se 2 [菱形,空间群R 32,a = 6.02813(13),c = 7.24883(16)Å,Z = 3]和NiSe [六角形,空间群P 6 3 / mmc,a的晶体结构 = 3.66147(10),c = 5.35766(16)Å,Z = 2]通过单晶X射线衍射分析并进行精制,分别得到117和85个唯一反射的R值分别为0.020和0.018,其中F o >4σ(F o)。R 32是其中存在对映体结构的Sohncke型空间群;通过-0.05(2)的Flack参数确认了通过细化获得的单域结构是正确的。除Ni-Se键外,在两种化合物中均证实了Ni-Ni键的存在。NiSe中每个原子的原子位移参数(均方位移)值大于Ni 3 Se 2中的原子位移参数(均方位移)值。NiSe中原子的更大振幅对应于NiSe中比Ni 3 Se 2中更长的Ni-Se和Ni-Ni键长。德拜温度,Gθ d,从观察到的均方位移为Ni和硒中的Ni估计3硒2,分别为322和298 K,分别,而对于Ni和硒NISE分别为246和241 K,分别。NiSe晶体的脆性和柔软性可能是由于结构中存在大的空穴以及弱的结合力所致。


























 京公网安备 11010802027423号
京公网安备 11010802027423号