Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A High‐Affinity Calmodulin‐Binding Site in the CyaA Toxin Translocation Domain is Essential for Invasion of Eukaryotic Cells
Advanced Science ( IF 15.1 ) Pub Date : 2021-03-08 , DOI: 10.1002/advs.202003630 Alexis Voegele 1, 2 , Mirko Sadi 1, 2 , Darragh Patrick O'Brien 1 , Pauline Gehan 3 , Dorothée Raoux-Barbot 1 , Maryline Davi 1 , Sylviane Hoos 4 , Sébastien Brûlé 4 , Bertrand Raynal 4 , Patrick Weber 5 , Ariel Mechaly 5 , Ahmed Haouz 5 , Nicolas Rodriguez 3 , Patrice Vachette 6 , Dominique Durand 6 , Sébastien Brier 7 , Daniel Ladant 1 , Alexandre Chenal 1
Advanced Science ( IF 15.1 ) Pub Date : 2021-03-08 , DOI: 10.1002/advs.202003630 Alexis Voegele 1, 2 , Mirko Sadi 1, 2 , Darragh Patrick O'Brien 1 , Pauline Gehan 3 , Dorothée Raoux-Barbot 1 , Maryline Davi 1 , Sylviane Hoos 4 , Sébastien Brûlé 4 , Bertrand Raynal 4 , Patrick Weber 5 , Ariel Mechaly 5 , Ahmed Haouz 5 , Nicolas Rodriguez 3 , Patrice Vachette 6 , Dominique Durand 6 , Sébastien Brier 7 , Daniel Ladant 1 , Alexandre Chenal 1
Affiliation
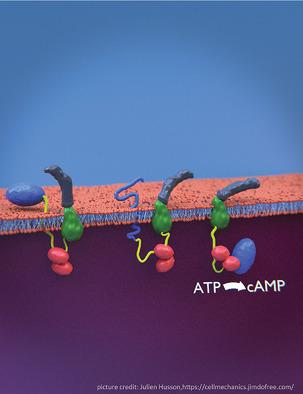
|
The molecular mechanisms and forces involved in the translocation of bacterial toxins into host cells are still a matter of intense research. The adenylate cyclase (CyaA) toxin from Bordetella pertussis displays a unique intoxication pathway in which its catalytic domain is directly translocated across target cell membranes. The CyaA translocation region contains a segment, P454 (residues 454–484), which exhibits membrane‐active properties related to antimicrobial peptides. Herein, the results show that this peptide is able to translocate across membranes and to interact with calmodulin (CaM). Structural and biophysical analyses reveal the key residues of P454 involved in membrane destabilization and calmodulin binding. Mutational analysis demonstrates that these residues play a crucial role in CyaA translocation into target cells. In addition, calmidazolium, a calmodulin inhibitor, efficiently blocks CyaA internalization. It is proposed that after CyaA binding to target cells, the P454 segment destabilizes the plasma membrane, translocates across the lipid bilayer and binds calmodulin. Trapping of CyaA by the CaM:P454 interaction in the cytosol may assist the entry of the N‐terminal catalytic domain by converting the stochastic motion of the polypeptide chain through the membrane into an efficient vectorial chain translocation into host cells.
中文翻译:

CyaA 毒素易位结构域中的高亲和力钙调蛋白结合位点对于入侵真核细胞至关重要
涉及细菌毒素向宿主细胞易位的分子机制和力量仍然是一个深入研究的问题。来自百日咳博德特氏菌的腺苷酸环化酶 (CyaA) 毒素显示出一种独特的中毒途径,其中其催化结构域直接跨靶细胞膜易位。CyaA 易位区包含一个片段 P454(残基 454-484),它表现出与抗菌肽相关的膜活性特性。在此,结果表明该肽能够跨膜易位并与钙调蛋白 (CaM) 相互作用。结构和生物物理分析揭示了 P454 的关键残基,这些残基与膜不稳定和钙调素结合有关。突变分析表明这些残基在 CyaA 易位到靶细胞中起关键作用。此外,钙调素抑制剂钙咪唑可以有效阻断 CyaA 内化。有人提出,在 CyaA 与靶细胞结合后,P454 片段使质膜不稳定,跨脂质双层转运并结合钙调素。通过胞质溶胶中的 CaM:P454 相互作用捕获 CyaA 可以通过将多肽链通过膜的随机运动转化为有效的载体链易位进入宿主细胞,从而帮助 N 端催化结构域的进入。
更新日期:2021-05-05
中文翻译:

CyaA 毒素易位结构域中的高亲和力钙调蛋白结合位点对于入侵真核细胞至关重要
涉及细菌毒素向宿主细胞易位的分子机制和力量仍然是一个深入研究的问题。来自百日咳博德特氏菌的腺苷酸环化酶 (CyaA) 毒素显示出一种独特的中毒途径,其中其催化结构域直接跨靶细胞膜易位。CyaA 易位区包含一个片段 P454(残基 454-484),它表现出与抗菌肽相关的膜活性特性。在此,结果表明该肽能够跨膜易位并与钙调蛋白 (CaM) 相互作用。结构和生物物理分析揭示了 P454 的关键残基,这些残基与膜不稳定和钙调素结合有关。突变分析表明这些残基在 CyaA 易位到靶细胞中起关键作用。此外,钙调素抑制剂钙咪唑可以有效阻断 CyaA 内化。有人提出,在 CyaA 与靶细胞结合后,P454 片段使质膜不稳定,跨脂质双层转运并结合钙调素。通过胞质溶胶中的 CaM:P454 相互作用捕获 CyaA 可以通过将多肽链通过膜的随机运动转化为有效的载体链易位进入宿主细胞,从而帮助 N 端催化结构域的进入。



























 京公网安备 11010802027423号
京公网安备 11010802027423号