当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
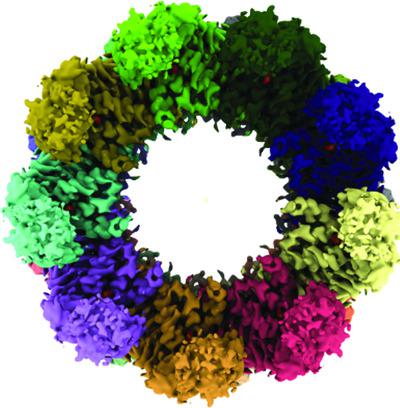
Structural analysis of the Sulfolobus solfataricus TF55β chaperonin by cryo‐electron microscopy
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2021-03-08 , DOI: 10.1107/s2053230x21002223 Yi Cheng Zeng 1 , Meghna Sobti 1 , Alastair G Stewart 1
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2021-03-08 , DOI: 10.1107/s2053230x21002223 Yi Cheng Zeng 1 , Meghna Sobti 1 , Alastair G Stewart 1
Affiliation
|
Chaperonins are biomolecular complexes that assist in protein folding. Thermophilic factor 55 (TF55) is a group II chaperonin found in the archaeal genus Sulfolobus that has α, β and γ subunits. Using cryo‐electron microscopy, structures of the β‐only complex of S. solfataricus TF55 (TF55β) were determined to 3.6–4.2 Å resolution. The structures of the TF55β complexes formed in the presence of ADP or ATP highlighted an open state in which nucleotide exchange can occur before progressing in the refolding cycle.
中文翻译:

低温电子显微镜对硫磺硫化叶菌 TF55β 伴侣蛋白的结构分析
伴侣蛋白是协助蛋白质折叠的生物分子复合物。嗜热因子 55 (TF55) 是在古菌属硫化叶菌中发现的 II 类伴侣蛋白,具有 α、β 和 γ 亚基。使用冷冻电子显微镜,以 3.6-4.2 Å 的分辨率确定了S. solfataricus TF55 (TF55β) 的仅 β 复合物的结构。在 ADP 或 ATP 存在下形成的 TF55β 复合物的结构突出了一种开放状态,在这种状态下,在进行重折叠周期之前可以发生核苷酸交换。
更新日期:2021-03-08
中文翻译:

低温电子显微镜对硫磺硫化叶菌 TF55β 伴侣蛋白的结构分析
伴侣蛋白是协助蛋白质折叠的生物分子复合物。嗜热因子 55 (TF55) 是在古菌属硫化叶菌中发现的 II 类伴侣蛋白,具有 α、β 和 γ 亚基。使用冷冻电子显微镜,以 3.6-4.2 Å 的分辨率确定了S. solfataricus TF55 (TF55β) 的仅 β 复合物的结构。在 ADP 或 ATP 存在下形成的 TF55β 复合物的结构突出了一种开放状态,在这种状态下,在进行重折叠周期之前可以发生核苷酸交换。


























 京公网安备 11010802027423号
京公网安备 11010802027423号