当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Band Gap as a Novel Descriptor for the Reactivity of 2D Titanium Dioxide and its Supported Pt Single Atom for Methane Activation
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2021-03-05 , DOI: 10.1021/acs.jpclett.1c00318 Xianglan Xu 1, 2 , Xiang Wang 1 , De-en Jiang 2
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2021-03-05 , DOI: 10.1021/acs.jpclett.1c00318 Xianglan Xu 1, 2 , Xiang Wang 1 , De-en Jiang 2
Affiliation
|
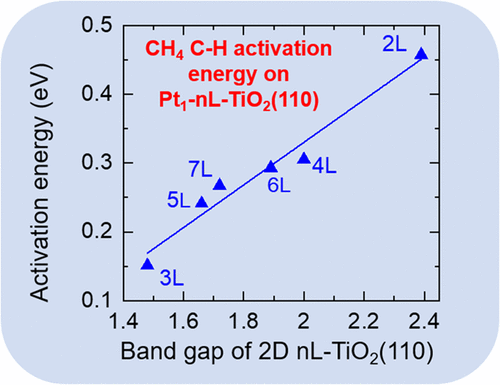
Ultrathin transition-metal oxides (TMOs) from nonlayered bulk structures are emerging 2D materials. Here we investigate the reactivity of a 2D TMO of varying thickness from first principles. We find that the band gap of the 2D nL-TiO2(110) shows a strong linear correlation with its surface reactivity: the smaller the band gap, the more reactive the surface oxygen; 3L-TiO2(110) has the smallest band gap and the highest reactivity. We further design Pt1 single-atom catalysts (SAC) by substituting a Pt single atom for a surface Ti atom. We find that the band gap of nL-TiO2(110) dictates both chemisorption and dissociation of CH4 on Pt1-nL-TiO2(110): the smaller the band gap, the stronger the adsorption of CH4 and the lower the barrier of heterolytic C–H activation of CH4. We propose that band gap can be a novel and direct descriptor for the reactivity of 2D TMOs and their supported SACs.
中文翻译:

带隙作为二维二氧化钛及其支持的Pt单原子对甲烷活化反应性的新型描述子
来自非分层体结构的超薄过渡金属氧化物(TMO)是新兴的2D材料。在这里,我们从第一原理出发,研究了厚度变化的2D TMO的反应性。我们发现2D n L-TiO 2(110)的带隙与它的表面反应性显示出很强的线性关系:带隙越小,表面氧的反应性越强;3L-TiO 2(110)具有最小的带隙和最高的反应性。我们通过用Pt单原子替代表面Ti原子来进一步设计Pt 1单原子催化剂(SAC)。我们发现n L-TiO 2(110)的带隙决定了Pt 1上CH 4的化学吸附和解离- Ñ L-的TiO 2(110):带隙越小,越强CH的吸附4和下部异裂CH活化CH的屏障4。我们建议带隙可以是2D TMO及其支持的SAC的反应性的新颖和直接的描述符。
更新日期:2021-03-18
中文翻译:

带隙作为二维二氧化钛及其支持的Pt单原子对甲烷活化反应性的新型描述子
来自非分层体结构的超薄过渡金属氧化物(TMO)是新兴的2D材料。在这里,我们从第一原理出发,研究了厚度变化的2D TMO的反应性。我们发现2D n L-TiO 2(110)的带隙与它的表面反应性显示出很强的线性关系:带隙越小,表面氧的反应性越强;3L-TiO 2(110)具有最小的带隙和最高的反应性。我们通过用Pt单原子替代表面Ti原子来进一步设计Pt 1单原子催化剂(SAC)。我们发现n L-TiO 2(110)的带隙决定了Pt 1上CH 4的化学吸附和解离- Ñ L-的TiO 2(110):带隙越小,越强CH的吸附4和下部异裂CH活化CH的屏障4。我们建议带隙可以是2D TMO及其支持的SAC的反应性的新颖和直接的描述符。


























 京公网安备 11010802027423号
京公网安备 11010802027423号