当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Systematic Analysis of the Role of Substituents in Oxiranes, Oxetanes, and Oxathietanes Chemical Shifts
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2021-03-04 , DOI: 10.1021/acs.jpca.0c10642 Angelika Baranowska-Łączkowska 1 , Krzysztof Z. Łączkowski 2 , Agnieszka Banaszak-Piechowska 1 , Berta Fernández 3
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2021-03-04 , DOI: 10.1021/acs.jpca.0c10642 Angelika Baranowska-Łączkowska 1 , Krzysztof Z. Łączkowski 2 , Agnieszka Banaszak-Piechowska 1 , Berta Fernández 3
Affiliation
|
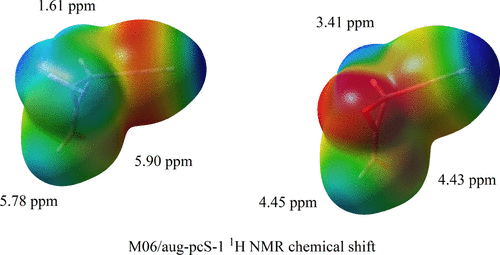
The important role substituents play on proton chemical shifts in heterocyclic compounds was investigated in detail. For this purpose, a considerable number of model oxiranes, oxetanes, and oxathietanes with different substituents were studied in a systematic way. In addition, the oxygen and sulfur heteroatom influence on the chemical shift values was analyzed. The density functional theory (DFT) approximation was employed together with the M06 and the B3LYP functionals and the aug-pcS-1 and the 6-311++G** basis sets. We carried out a careful analysis of the shift values and the changes in the corresponding molecular electrostatic potential surfaces due to substitution. We observed that chemical shift values for the protons closest to the substituents are larger for the chloro and fluoro derivatives than those for the cyano and ethynyl ones. The presence of oxygen as well as sulfur in the ring causes an increase of the chemical shift values, most pronounced for the atom closest to the substituent. A large decrease of the proton shifts was observed when going from methylenecyclopropane to methyleneoxirane that can be attributed to π-electron resonance. Protons diagonal to the substituents behaved in a different way depending on their cis or trans disposition with respect to them. The conclusions of the present study will be useful in theoretical and experimental work on NMR spectra of heterocyclic compounds.
中文翻译:

系统分析环氧乙烷,环氧乙烷和环氧乙烷类化学位移中取代基的作用
详细研究了取代基对杂环化合物中质子化学位移的重要作用。为了这个目的,以系统的方式研究了大量的具有不同取代基的模型氧杂环丁烷,氧杂环丁烷和氧杂环丁烷。另外,分析了氧和硫杂原子对化学位移值的影响。密度泛函理论(DFT)近似与M06和B3LYP泛函以及aug-pcS-1和6-311 ++ G **基集一起使用。我们对位移值以及由于取代引起的相应分子静电势面的变化进行了仔细的分析。我们观察到,最接近取代基的质子的化学位移值对于氯代和氟代衍生物要比氰基和乙炔基更大。环中氧以及硫的存在引起化学位移值的增加,这对于最接近取代基的原子最为明显。当从亚甲基环丙烷变为亚甲基环氧乙烷时,观察到质子位移大大降低,这可归因于π电子共振。与取代基对角的质子根据它们的顺式或反式排列而表现出不同的方式。本研究的结论将对杂环化合物的NMR光谱的理论和实验工作有用。当从亚甲基环丙烷变为亚甲基环氧乙烷时,观察到质子位移大大降低,这可归因于π电子共振。与取代基对角的质子根据它们的顺式或反式排列以不同的方式表现。本研究的结论将对杂环化合物的NMR光谱的理论和实验工作有用。当从亚甲基环丙烷变为亚甲基环氧乙烷时,观察到质子位移大大降低,这可归因于π电子共振。与取代基对角的质子根据它们的顺式或反式排列以不同的方式表现。本研究的结论将对杂环化合物的NMR光谱的理论和实验工作有用。
更新日期:2021-03-18
中文翻译:

系统分析环氧乙烷,环氧乙烷和环氧乙烷类化学位移中取代基的作用
详细研究了取代基对杂环化合物中质子化学位移的重要作用。为了这个目的,以系统的方式研究了大量的具有不同取代基的模型氧杂环丁烷,氧杂环丁烷和氧杂环丁烷。另外,分析了氧和硫杂原子对化学位移值的影响。密度泛函理论(DFT)近似与M06和B3LYP泛函以及aug-pcS-1和6-311 ++ G **基集一起使用。我们对位移值以及由于取代引起的相应分子静电势面的变化进行了仔细的分析。我们观察到,最接近取代基的质子的化学位移值对于氯代和氟代衍生物要比氰基和乙炔基更大。环中氧以及硫的存在引起化学位移值的增加,这对于最接近取代基的原子最为明显。当从亚甲基环丙烷变为亚甲基环氧乙烷时,观察到质子位移大大降低,这可归因于π电子共振。与取代基对角的质子根据它们的顺式或反式排列而表现出不同的方式。本研究的结论将对杂环化合物的NMR光谱的理论和实验工作有用。当从亚甲基环丙烷变为亚甲基环氧乙烷时,观察到质子位移大大降低,这可归因于π电子共振。与取代基对角的质子根据它们的顺式或反式排列以不同的方式表现。本研究的结论将对杂环化合物的NMR光谱的理论和实验工作有用。当从亚甲基环丙烷变为亚甲基环氧乙烷时,观察到质子位移大大降低,这可归因于π电子共振。与取代基对角的质子根据它们的顺式或反式排列以不同的方式表现。本研究的结论将对杂环化合物的NMR光谱的理论和实验工作有用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号