当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mizoroki–Heck Reaction of Unstrained Aryl Ketones via Ligand-Promoted C–C Bond Olefination
Organic Letters ( IF 5.2 ) Pub Date : 2021-03-04 , DOI: 10.1021/acs.orglett.1c00296 Mei-Ling Wang 1 , Hui Xu 2 , Han-Yuan Li 2 , Biao Ma 2 , Zhen-Yu Wang 3 , Xing Wang 2 , Hui-Xiong Dai 2, 4
Organic Letters ( IF 5.2 ) Pub Date : 2021-03-04 , DOI: 10.1021/acs.orglett.1c00296 Mei-Ling Wang 1 , Hui Xu 2 , Han-Yuan Li 2 , Biao Ma 2 , Zhen-Yu Wang 3 , Xing Wang 2 , Hui-Xiong Dai 2, 4
Affiliation
|
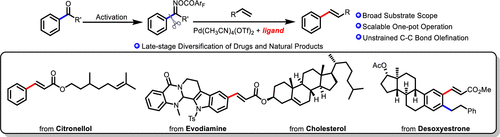
Mizoroki–Heck reaction of unstrained aryl ketone with acrylate/styrene is accomplished via palladium-catalyzed ligand-promoted C–C bond cleavage. Various (hetero)aryl ketones are compatible in the reaction, affording the alkene product in good to excellent yields. Further applications in the late-stage olefination of some drugs, natural products, and fragrance-derived aryl ketones demonstrate the synthetic utility of this protocol. By employing ketone as both the directing group and the leaving group, 1,2-bifunctionalization is achieved via sequential ortho-C–H alkylation/ipso-Heck olefination.
中文翻译:

通过配体促进的C–C键渗碳反应实现未过滤芳基酮的Mizoroki–Heck反应
未应变的芳基酮与丙烯酸酯/苯乙烯的Mizoroki-Heck反应是通过钯催化的配体促进的C-C键裂解实现的。各种(杂)芳基酮在反应中是相容的,从而以良好至优异的产率提供了烯烃产物。在某些药物,天然产物和香料衍生的芳基酮的后期烯烃聚合中的进一步应用证明了该方案的合成效用。通过采用酮作为导向基团和离去基团二者,1,2- bifunctionalization经由顺序实现邻-C-H的烷基化/本位-Heck烯。
更新日期:2021-03-19
中文翻译:

通过配体促进的C–C键渗碳反应实现未过滤芳基酮的Mizoroki–Heck反应
未应变的芳基酮与丙烯酸酯/苯乙烯的Mizoroki-Heck反应是通过钯催化的配体促进的C-C键裂解实现的。各种(杂)芳基酮在反应中是相容的,从而以良好至优异的产率提供了烯烃产物。在某些药物,天然产物和香料衍生的芳基酮的后期烯烃聚合中的进一步应用证明了该方案的合成效用。通过采用酮作为导向基团和离去基团二者,1,2- bifunctionalization经由顺序实现邻-C-H的烷基化/本位-Heck烯。


























 京公网安备 11010802027423号
京公网安备 11010802027423号