当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-assembly of an imidazolium surfactant in aprotic ionic liquids. 2. More than solvents
Soft Matter ( IF 3.4 ) Pub Date : 2021-2-10 , DOI: 10.1039/d1sm00039j Wenchang Zhuang 1, 2, 3, 4 , Chunhua Zhao 4, 5, 6, 6, 7 , Yue Pan 1, 2, 3, 4 , Qintang Li 1, 2, 3, 4
Soft Matter ( IF 3.4 ) Pub Date : 2021-2-10 , DOI: 10.1039/d1sm00039j Wenchang Zhuang 1, 2, 3, 4 , Chunhua Zhao 4, 5, 6, 6, 7 , Yue Pan 1, 2, 3, 4 , Qintang Li 1, 2, 3, 4
Affiliation
|
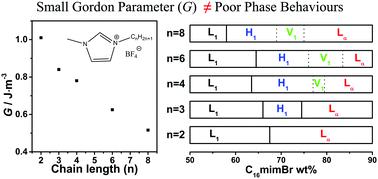
As tailorable solvents, the physiochemical properties of ionic liquids can be tuned by the structure of ions. Herein, we investigate the structural effects of ILs on the self-assembly of surfactants. It has been confirmed that the cationic surfactant 1-hexadecyl-3-methylimidazolium bromide (C16mimBr) can self-assemble into micellar and lamellar lyotropic liquid crystal phases in the aprotic ionic liquid (AIL) 1-ethyl-3-methylimidazolium tetrafluoroborate ([Emim]BF4). In this work, we explore the aggregation behaviours in AILs with different alkyl chains on the imidazolium group, i.e., 1-propyl-3-methylimidazolium tetrafluoroborate ([Pmim]BF4), 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4), 1-hexyl-3-methylimidazolium tetrafluoroborate ([Hmim]BF4) and 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim]BF4). With the increase of the cation chain length, AILs have better solubility of the solvophobic part of the surfactants and hence a weaker driving force for self-assembly. Therefore, the critical micellization concentration of C16mimBr in AILs increases as confirmed by the surface tension and small angle X-ray scattering characterizations. More interesting things happen to the phase behaviours. Besides the micellar and lamellar lyotropic liquid crystal phases, a hexagonal lyotropic liquid crystal phase is formed in [Pmim]BF4 while hexagonal and bicontinuous cubic lyotropic liquid crystal phases are formed in [Bmim]BF4, [Hmim]BF4 and [Omim]BF4. It is surprising to observe richer phase behaviours in solvents of lower cohesive energy. The detailed structural information of various aggregates has been obtained by small-angle X-ray scattering. It is demonstrated that AILs work as not only solvents but also co-surfactants.
中文翻译:

咪唑鎓表面活性剂在非质子离子液体中的自组装。2.比溶剂更多
作为可调节的溶剂,离子液体的物理化学性质可以通过离子的结构进行调整。在这里,我们研究表面活性剂对表面活性剂自组装的结构影响。已证实阳离子表面活性剂溴化1-十六烷基-3-甲基咪唑鎓(C 16 mimBr)可以在非质子离子液体(AIL)1-乙基-3-甲基咪唑鎓四氟硼酸盐( [Emim] BF 4)。在这项工作中,我们探索在咪唑基团上具有不同烷基链的AIL中的聚集行为,即1-丙基-3-甲基咪唑四氟硼酸酯([Pmim] BF 4),1-丁基-3-甲基咪唑四氟硼酸酯([Bmim]高炉4),1-己基-3-甲基咪唑四氟硼酸酯([Hmim] BF 4)和1-辛基-3-甲基咪唑四氟硼酸酯([Omim] BF 4)。随着阳离子链长度的增加,AIL在表面活性剂的疏溶剂部分具有更好的溶解性,因此对自组装的驱动力更弱。因此,如表面张力和小角度X射线散射特征所证实的,AIL中C 16 mimBr的临界胶束浓度增加。更有趣的事情发生在阶段行为上。除了胶束和层状溶致液晶相之外,在[Pmim] BF 4中还形成了六方溶致液晶相。在[Bmim] BF 4,[Hmim] BF 4和[Omim] BF 4中形成六方和双连续立方溶致液晶相。令人惊讶的是,在较低内聚能的溶剂中观察到更丰富的相行为。各种聚集体的详细结构信息已通过小角度X射线散射获得。事实证明,AILs不仅可以作为溶剂,而且可以作为辅助表面活性剂。
更新日期:2021-03-04
中文翻译:

咪唑鎓表面活性剂在非质子离子液体中的自组装。2.比溶剂更多
作为可调节的溶剂,离子液体的物理化学性质可以通过离子的结构进行调整。在这里,我们研究表面活性剂对表面活性剂自组装的结构影响。已证实阳离子表面活性剂溴化1-十六烷基-3-甲基咪唑鎓(C 16 mimBr)可以在非质子离子液体(AIL)1-乙基-3-甲基咪唑鎓四氟硼酸盐( [Emim] BF 4)。在这项工作中,我们探索在咪唑基团上具有不同烷基链的AIL中的聚集行为,即1-丙基-3-甲基咪唑四氟硼酸酯([Pmim] BF 4),1-丁基-3-甲基咪唑四氟硼酸酯([Bmim]高炉4),1-己基-3-甲基咪唑四氟硼酸酯([Hmim] BF 4)和1-辛基-3-甲基咪唑四氟硼酸酯([Omim] BF 4)。随着阳离子链长度的增加,AIL在表面活性剂的疏溶剂部分具有更好的溶解性,因此对自组装的驱动力更弱。因此,如表面张力和小角度X射线散射特征所证实的,AIL中C 16 mimBr的临界胶束浓度增加。更有趣的事情发生在阶段行为上。除了胶束和层状溶致液晶相之外,在[Pmim] BF 4中还形成了六方溶致液晶相。在[Bmim] BF 4,[Hmim] BF 4和[Omim] BF 4中形成六方和双连续立方溶致液晶相。令人惊讶的是,在较低内聚能的溶剂中观察到更丰富的相行为。各种聚集体的详细结构信息已通过小角度X射线散射获得。事实证明,AILs不仅可以作为溶剂,而且可以作为辅助表面活性剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号