Chemical Engineering Journal ( IF 15.1 ) Pub Date : 2021-03-04 , DOI: 10.1016/j.cej.2021.129205 Xia Zhou , Fernando Plascencia-Hernández , Feng Yu , Heriberto Pfeiffer
|
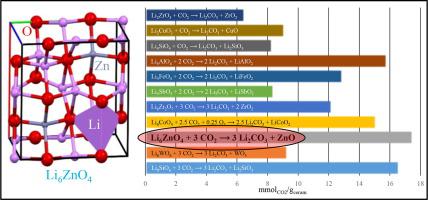
Li6ZnO4 was synthesized, structural and microstructurally characterized and tested as possible carbon dioxide (CO2) chemical captor. The structural analysis suggests that Li6ZnO4 may have high Li-ion diffusion, based on its crystal triangle gap area measurement, which is among the highest ones analyzed within this context. Thus, Li6ZnO4 was dynamic and isothermally tested under different CO2 partial pressures (1.0 and 0.2) in the presence or absence of oxygen, through thermogravimetric techniques. Li6ZnO4 exhibited excellent CO2 chemisorption efficiencies, even when the CO2 partial pressure was as low as 0.2. Moreover, the kinetic analyzed was performed using the modified Jander-Zhang model, showing fast and efficient CO2 carbonation, where the kinetics enhanced as a function of the CO2 concentration, as it would be expected. The XRD analysis of the isothermal products allowed to determine the CO2 chemisorption reaction path, where an intermediate Li10Zn4O9 crystal phase was observed. Thus, a topochemical lithium release was assumed to explain the whole Li6ZnO4 carbonation process, supported by the crystal structure analysis. Additionally, a cyclic Li6ZnO4 carbonation-decarbonation test evidenced interesting efficiencies, of around 60.0% after 10 cycles.
中文翻译:

了解新的可能的高温CO 2捕获剂Li 6 ZnO 4上的CO 2化学反应路径
合成了Li 6 ZnO 4,对其结构和微观结构进行了表征,并测试了其作为二氧化碳(CO 2)化学捕获剂的能力。结构分析表明,基于其晶体三角形间隙面积测量,Li 6 ZnO 4可能具有较高的Li离子扩散,这是在此背景下分析得出的最高值。因此,通过热重技术,在存在或不存在氧气的情况下,在不同的CO 2分压(1.0和0.2)下,对Li 6 ZnO 4进行了动态和等温测试。Li 6 ZnO 4表现出优异的CO 2化学吸附效率,即使当CO 2分压低至0.2时也是如此。此外,使用改进的Jander-Zhang模型进行动力学分析,显示出快速而有效的CO 2碳酸化,正如预期的那样,动力学随CO 2浓度的变化而增强。等温产物的XRD分析可以确定CO 2化学吸附反应路径,其中观察到中间的Li 10 Zn 4 O 9晶相。因此,假定释放了化学锂来解释整个Li 6 ZnO 4碳酸化过程,由晶体结构分析支持。另外,循环的Li 6 ZnO 4碳化-脱碳测试显示出令人感兴趣的效率,在10个循环后,效率约为60.0%。


























 京公网安备 11010802027423号
京公网安备 11010802027423号