当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mutual structural effects of unmodified and pyroglutamylated amyloid β peptides during aggregation
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2021-02-25 , DOI: 10.1002/psc.3312 Faisal Abedin 1 , Suren A Tatulian 2
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2021-02-25 , DOI: 10.1002/psc.3312 Faisal Abedin 1 , Suren A Tatulian 2
Affiliation
|
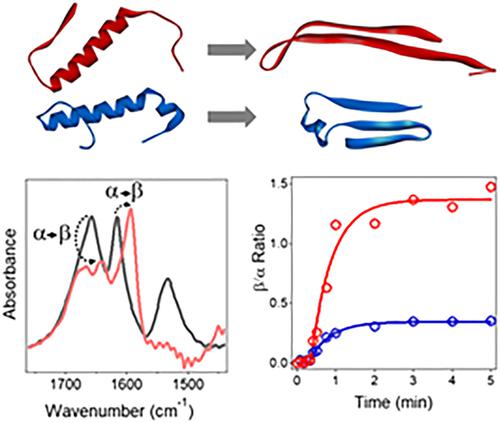
Amyloid β (Aβ) peptide aggregates are linked to Alzheimer's disease (AD). Posttranslationally pyroglutamylated Aβ (pEAβ) occurs in AD brains in significant quantities and is hypertoxic, but the underlying structural and aggregation properties remain poorly understood. Here, the structure and aggregation of Aβ1–40 and pEAβ3–40 are analyzed separately and in equimolar combination. Circular dichroism data show that Aβ1–40, pEAβ3–40, and their combination assume α‐helical structure in dry state and transition to unordered structure in aqueous buffer. Aβ1–40 and the 1:1 combination gradually acquire β‐sheet structure while pEAβ3–40 adopts an α‐helix/β‐sheet conformation. Thioflavin‐T fluorescence studies suggest that the two peptides mutually inhibit fibrillogenesis. Fourier transform infrared (FTIR) spectroscopy identifies the presence of β‐turn and α‐helical structures in addition to β‐sheet structure in peptides in aqueous buffer. The kinetics of transitions from the initial α‐helical structure to β‐sheet structure were resolved by slow hydration of dry peptides by D2O vapor, coupled with isotope‐edited FTIR. These data confirmed the mutual suppression of β‐sheet formation by the two peptides. Remarkably, pEAβ3–40 maintained a significant fraction of α‐helical structure in the combined sample, implying a reduced β‐sheet propensity of pEAβ3–40. Altogether, the data imply that the combination of unmodified and pyroglutamylated Aβ peptides resists fibrillogenesis and favors the prefibrillar state, which may underlie hypertoxicity of pEAβ.
中文翻译:

未修饰和焦谷氨酰化淀粉样 β 肽在聚集过程中的相互结构影响
淀粉样蛋白 β (Aβ) 肽聚集体与阿尔茨海默病 (AD) 相关。翻译后焦谷氨酰化 Aβ (pEAβ) 大量存在于 AD 大脑中并且具有剧毒,但其潜在的结构和聚集特性仍知之甚少。在这里,Aβ 1-40和 pEAβ 3-40的结构和聚集被分别和等摩尔组合分析。圆二色性数据表明,Aβ 1-40、pEAβ 3-40及其组合在干燥状态下呈现α-螺旋结构,并在水性缓冲液中转变为无序结构。Aβ 1-40和 1:1 组合逐渐获得 β-折叠结构,而 pEAβ 3-40采用α-螺旋/β-折叠构象。Thioflavin-T 荧光研究表明这两种肽相互抑制原纤维生成。傅里叶变换红外 (FTIR) 光谱确定了水性缓冲液中肽中除 β-折叠结构外还存在 β-转角和 α-螺旋结构。从初始α-螺旋结构到β-折叠结构的转变动力学通过D 2 O蒸汽对干肽的缓慢水合以及同位素编辑的FTIR来解决。这些数据证实了两种肽对β-折叠形成的相互抑制。值得注意的是,pEAβ 3-40在组合样品中保留了很大一部分α-螺旋结构,这意味着 pEAβ 3-40 的β-折叠倾向降低. 总而言之,数据暗示未修饰和焦谷氨酰化 Aβ 肽的组合抵抗原纤维形成并有利于前原纤维状态,这可能是 pEAβ 毒性的基础。
更新日期:2021-04-29
中文翻译:

未修饰和焦谷氨酰化淀粉样 β 肽在聚集过程中的相互结构影响
淀粉样蛋白 β (Aβ) 肽聚集体与阿尔茨海默病 (AD) 相关。翻译后焦谷氨酰化 Aβ (pEAβ) 大量存在于 AD 大脑中并且具有剧毒,但其潜在的结构和聚集特性仍知之甚少。在这里,Aβ 1-40和 pEAβ 3-40的结构和聚集被分别和等摩尔组合分析。圆二色性数据表明,Aβ 1-40、pEAβ 3-40及其组合在干燥状态下呈现α-螺旋结构,并在水性缓冲液中转变为无序结构。Aβ 1-40和 1:1 组合逐渐获得 β-折叠结构,而 pEAβ 3-40采用α-螺旋/β-折叠构象。Thioflavin-T 荧光研究表明这两种肽相互抑制原纤维生成。傅里叶变换红外 (FTIR) 光谱确定了水性缓冲液中肽中除 β-折叠结构外还存在 β-转角和 α-螺旋结构。从初始α-螺旋结构到β-折叠结构的转变动力学通过D 2 O蒸汽对干肽的缓慢水合以及同位素编辑的FTIR来解决。这些数据证实了两种肽对β-折叠形成的相互抑制。值得注意的是,pEAβ 3-40在组合样品中保留了很大一部分α-螺旋结构,这意味着 pEAβ 3-40 的β-折叠倾向降低. 总而言之,数据暗示未修饰和焦谷氨酰化 Aβ 肽的组合抵抗原纤维形成并有利于前原纤维状态,这可能是 pEAβ 毒性的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号