Aquatic Toxicology ( IF 4.5 ) Pub Date : 2021-02-25 , DOI: 10.1016/j.aquatox.2021.105794 Corinna Singleman , Nathalia G. Holtzman
|
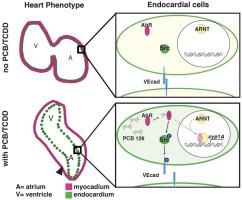
Polychlorinated biphenyls (PCBs) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are environmental contaminants known to impact cardiac development, a key step in the embryonic development of most animals. To date, little is understood of the molecular mechanism driving the observed cardiac defects in exposed fishes. The literature shows PCB & TCDD derived cardiac defects are concurrent with, but not caused by, expression of cyp1A, due to activation of the aryl hydrocarbon receptor (AhR) gene activation pathway. However, in this study, detailed visualization of fish hearts exposed to PCBs and TCDD show that, in addition to a failure of cardiac looping in early heart development, the inner endocardial lining of the heart fails to maintain proper cell adhesion and tissue integrity. The resulting gap between the endocardium and myocardium in both zebrafish and Atlantic sturgeon suggested functional faults in endothelial adherens junction formation. Thus, we explored the molecular mechanism triggering cardiac defects using immunohistochemistry to identify the location and phosphorylation state of key regulatory and adhesion molecules. We hypothesized that PCB and TCDD activates AhR, phosphorylating Src, which then phosphorylates the endothelial adherens junction protein, VEcadherin. When phosphorylated, VEcadherin dimers, found in the endocardium and vasculature, separate, reducing tissue integrity. In zebrafish, treatment with PCB and TCDD contaminants leads to higher phosphorylation of VEcadherin in cardiac tissue suggesting that these cells have reduced connectivity. Small molecule inhibition of Src phosphorylation prevents contaminant stimulated phosphorylation of VEcadherin and rescues both cardiac function and gross morphology. Atlantic sturgeon hearts show parallels to contaminant exposed zebrafish cardiac phenotype at the tissue level. These data suggest that the mechanism for PCB and TCDD action in the heart is, in part, distinct from the canonical mechanism described in the literature and that cardiac defects are impacted by this nongenomic mechanism.
中文翻译:

PCB和TCDD衍生的胚胎心脏缺陷源于新的AhR途径
多氯联苯(PCB)和2,3,7,8-四氯二苯并-p-二恶英(TCDD)是已知会影响心脏发育的环境污染物,这是大多数动物胚胎发育的关键步骤。迄今为止,对引起裸露鱼类中观察到的心脏缺陷的分子机制了解甚少。文献显示,PCB和TCDD衍生的心脏缺陷与cyp1A的表达同时发生,但并非由cyp1A的表达引起,这是由于芳基烃受体(AhR)基因激活途径的激活所致。然而,在这项研究中,对暴露于PCBs和TCDD的鱼心进行的详细可视化显示,除了心脏早期发育中的心脏循环失败之外,心脏的内部心内膜内衬无法维持适当的细胞粘附和组织完整性。斑马鱼和大西洋st鱼在心内膜和心肌之间产生的间隙提示内皮粘附连接形成中的功能性缺陷。因此,我们使用免疫组织化学探索了触发心脏缺陷的分子机制,以识别关键调节分子和粘附分子的位置和磷酸化状态。我们假设PCB和TCDD激活了AhR,使Src磷酸化,然后使内皮粘附连接蛋白VEcadherin磷酸化。当磷酸化时,在心内膜和脉管系统中发现的VEcadherin二聚体分离,从而降低了组织完整性。在斑马鱼中,用PCB和TCDD污染物处理可导致心脏组织中VEcadherin的磷酸化程度更高,表明这些细胞的连接性降低。Src磷酸化的小分子抑制作用可防止污染物刺激的VEcadherin磷酸化,并挽救心脏功能和总体形态。大西洋st鱼心脏在组织水平上与暴露于斑马鱼的污染物的心脏表型相似。这些数据表明,心脏中PCB和TCDD作用的机制与文献中描述的规范机制部分不同,并且心脏缺陷受这种非基因组机制的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号