Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Monitoring the neurotransmitter release of human midbrain organoids using a redox cycling microsensor as a novel tool for personalized Parkinson's disease modelling and drug screening
Analyst ( IF 4.2 ) Pub Date : 2021-2-17 , DOI: 10.1039/d0an02206c Cristian Zanetti 1, 2, 3, 4 , Sarah Spitz 1, 2, 3, 4 , Emanuel Berger 5, 6, 7, 8, 9 , Silvia Bolognin 5, 6, 7, 8, 9 , Lisa M. Smits 5, 6, 7, 8, 9 , Philipp Crepaz 1, 2, 3, 4 , Mario Rothbauer 1, 2, 3, 4 , Julie M. Rosser 1, 2, 3, 4 , Martina Marchetti-Deschmann 1, 2, 3, 4 , Jens C. Schwamborn 5, 6, 7, 8, 9 , Peter Ertl 1, 2, 3, 4
Analyst ( IF 4.2 ) Pub Date : 2021-2-17 , DOI: 10.1039/d0an02206c Cristian Zanetti 1, 2, 3, 4 , Sarah Spitz 1, 2, 3, 4 , Emanuel Berger 5, 6, 7, 8, 9 , Silvia Bolognin 5, 6, 7, 8, 9 , Lisa M. Smits 5, 6, 7, 8, 9 , Philipp Crepaz 1, 2, 3, 4 , Mario Rothbauer 1, 2, 3, 4 , Julie M. Rosser 1, 2, 3, 4 , Martina Marchetti-Deschmann 1, 2, 3, 4 , Jens C. Schwamborn 5, 6, 7, 8, 9 , Peter Ertl 1, 2, 3, 4
Affiliation
|
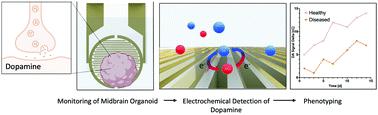
In this study, we have aimed at developing a novel electrochemical sensing approach capable of detecting dopamine, the main biomarker in Parkinson's disease, within the highly complex cell culture matrix of human midbrain organoids in a non-invasive and label-free manner. With its ability to generate organotypic structures in vitro, induced pluripotent stem cell technology has provided the basis for the development of advanced patient-derived disease models. These include models of the human midbrain, the affected region in the neurodegenerative disorder Parkinson's disease. Up to now, however, the analysis of so-called human midbrain organoids has relied on time-consuming and invasive strategies, incapable of monitoring organoid development. Using a redox-cycling approach in combination with a 3-mercaptopropionic acid self-assembled monolayer modification enabled the increase of sensor selectivity and sensitivity towards dopamine, while simultaneously reducing matrix-mediated interferences. In this work, we demonstrate the ability to detect and monitor even small differences in dopamine release between healthy and Parkinson`s disease-specific midbrain organoids over prolonged cultivation periods, which was additionally verified using liquid chromatography–multiple reaction monitoring mass spectrometry. Furthermore, the detection of a phenotypic rescue in midbrain organoids carrying a pathogenic mutation in leucine-rich repeat kinase 2, upon treatment with the leucine-rich repeat kinase 2 inhibitor II underlines the practical implementability of our sensing approach for drug screening applications as well as personalized disease modelling.
中文翻译:

使用氧化还原循环微传感器监测人中脑器官的神经递质释放,作为个性化帕金森氏病建模和药物筛选的新工具
在这项研究中,我们旨在开发一种新型的电化学传感方法,该方法能够以无创且无标记的方式在人中脑类器官的高度复杂的细胞培养基质中检测帕金森氏病的主要生物标志物多巴胺。具有在体外产生器官型结构的能力诱导多能干细胞技术为发展高级患者衍生疾病模型提供了基础。这些模型包括人类中脑模型,即神经退行性疾病帕金森氏病的患处。但是,到目前为止,对所谓的人类中脑类器官的分析都依赖于耗时且侵入性的策略,无法监测类器官的发育。将氧化还原循环方法与3-巯基丙酸自组装单层修饰结合使用,可以提高传感器的选择性和对多巴胺的敏感性,同时减少基质介导的干扰。在这项工作中,我们证明了在延长的培养时间内检测和监测健康人和帕金森氏病特定的中脑类器官中多巴胺释放的微小差异的能力,此外还使用液相色谱-多重反应监测质谱法进行了验证。此外,在使用富含亮氨酸的重复激酶2抑制剂II处理后,在富含亮氨酸的重复激酶2抑制剂II的致病性突变的中脑类器官中检测到表型拯救,突显了我们用于药物筛选应用的传感方法的实际可实施性,以及个性化疾病建模。
更新日期:2021-02-24
中文翻译:

使用氧化还原循环微传感器监测人中脑器官的神经递质释放,作为个性化帕金森氏病建模和药物筛选的新工具
在这项研究中,我们旨在开发一种新型的电化学传感方法,该方法能够以无创且无标记的方式在人中脑类器官的高度复杂的细胞培养基质中检测帕金森氏病的主要生物标志物多巴胺。具有在体外产生器官型结构的能力诱导多能干细胞技术为发展高级患者衍生疾病模型提供了基础。这些模型包括人类中脑模型,即神经退行性疾病帕金森氏病的患处。但是,到目前为止,对所谓的人类中脑类器官的分析都依赖于耗时且侵入性的策略,无法监测类器官的发育。将氧化还原循环方法与3-巯基丙酸自组装单层修饰结合使用,可以提高传感器的选择性和对多巴胺的敏感性,同时减少基质介导的干扰。在这项工作中,我们证明了在延长的培养时间内检测和监测健康人和帕金森氏病特定的中脑类器官中多巴胺释放的微小差异的能力,此外还使用液相色谱-多重反应监测质谱法进行了验证。此外,在使用富含亮氨酸的重复激酶2抑制剂II处理后,在富含亮氨酸的重复激酶2抑制剂II的致病性突变的中脑类器官中检测到表型拯救,突显了我们用于药物筛选应用的传感方法的实际可实施性,以及个性化疾病建模。



























 京公网安备 11010802027423号
京公网安备 11010802027423号