Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mapping Solvent Entrapment in Multiphase Systems by Electrogenerated Chemiluminescence
Langmuir ( IF 3.9 ) Pub Date : 2021-02-24 , DOI: 10.1021/acs.langmuir.0c03445 Matthew W. Glasscott 1 , Silvia Voci 1 , Philip J. Kauffmann 1 , Andrei I. Chapoval 2 , Jeffrey E. Dick 1, 3
Langmuir ( IF 3.9 ) Pub Date : 2021-02-24 , DOI: 10.1021/acs.langmuir.0c03445 Matthew W. Glasscott 1 , Silvia Voci 1 , Philip J. Kauffmann 1 , Andrei I. Chapoval 2 , Jeffrey E. Dick 1, 3
Affiliation
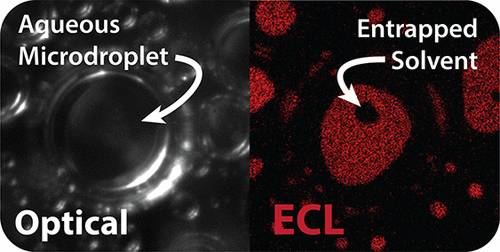
|
The interfacial properties of multiphase systems are often difficult to quantify. We describe the observation and quantification of immiscible solvent entrapment on a carbonaceous electrode surface using microscopy-coupled electrogenerated chemiluminescence (ECL). As aqueous microdroplets suspended in 1,2-dichloroethane collide with a glassy carbon electrode surface, small volumes of the solvent become entrapped between the electrode and aqueous phase, resulting in an overestimation of the true microdroplet/electrode contact area. To quantify the contribution of solvent entrapment decreasing the microdroplet contact area, we drive an ECL reaction within the microdroplet phase using tris(bipyridine)ruthenium(II) chloride ([Ru(bpy)3]Cl2) as the ECL luminophore and sodium oxalate (Na2C2O4) as the co-reactant. Importantly, the hydrophilicity of sodium oxalate ensures that the reaction proceeds in the aqueous phase, permitting a clear contrast between the aqueous and 1,2-dichloroethane present at the electrode interface. With the contrast provided by ECL imaging, we quantify the microdroplet radius, apparent microdroplet contact area (aqueous + entrapped 1,2-dichloroethane), entrapped solvent contact area, and the number of entrapped solvent pockets per droplet. These data permit the extraction of the true microdroplet/electrode contact area for a given droplet, as well as a statistical assessment regarding the probability of solvent entrapment based on microdroplet size.
中文翻译:

电生化学发光法分析多相系统中的溶剂截留
多相系统的界面特性通常难以量化。我们描述了观察和定量使用显微镜耦合电生化学发光(ECL)的碳质电极表面上不混溶的溶剂截留。当悬浮在1,2-二氯乙烷中的水性微滴与玻璃碳电极表面碰撞时,少量溶剂被截留在电极和水相之间,导致高估了真实的微滴/电极接触面积。为了量化溶剂截留减少微滴接触面积的贡献,我们使用三(联吡啶)钌(II)氯化物([Ru(bpy)3 ] Cl 2)作为ECL发光体和草酸钠驱动微滴相内的ECL反应(Na2 C 2 O 4)作为共反应物。重要的是,草酸钠的亲水性确保了反应在水相中进行,从而使水与电极界面处的1,2-二氯乙烷之间形成鲜明的对比。借助ECL成像提供的对比度,我们可以量化微滴半径,表观微滴接触面积(水+截留的1,2-二氯乙烷),截留的溶剂接触面积以及每滴截留的溶剂囊的数量。这些数据允许提取给定液滴的真实微滴/电极接触面积,并基于微滴大小进行有关溶剂截留概率的统计评估。
更新日期:2021-03-09
中文翻译:

电生化学发光法分析多相系统中的溶剂截留
多相系统的界面特性通常难以量化。我们描述了观察和定量使用显微镜耦合电生化学发光(ECL)的碳质电极表面上不混溶的溶剂截留。当悬浮在1,2-二氯乙烷中的水性微滴与玻璃碳电极表面碰撞时,少量溶剂被截留在电极和水相之间,导致高估了真实的微滴/电极接触面积。为了量化溶剂截留减少微滴接触面积的贡献,我们使用三(联吡啶)钌(II)氯化物([Ru(bpy)3 ] Cl 2)作为ECL发光体和草酸钠驱动微滴相内的ECL反应(Na2 C 2 O 4)作为共反应物。重要的是,草酸钠的亲水性确保了反应在水相中进行,从而使水与电极界面处的1,2-二氯乙烷之间形成鲜明的对比。借助ECL成像提供的对比度,我们可以量化微滴半径,表观微滴接触面积(水+截留的1,2-二氯乙烷),截留的溶剂接触面积以及每滴截留的溶剂囊的数量。这些数据允许提取给定液滴的真实微滴/电极接触面积,并基于微滴大小进行有关溶剂截留概率的统计评估。


























 京公网安备 11010802027423号
京公网安备 11010802027423号