Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the Mechanism of Antibody-Triggered Aggregation of Gold Nanoparticles
Langmuir ( IF 3.9 ) Pub Date : 2021-02-23 , DOI: 10.1021/acs.langmuir.1c00100 Samuel Okyem 1 , Olatunde Awotunde 1 , Tosin Ogunlusi 1 , McKenzie B Riley 1 , Jeremy D Driskell 1
Langmuir ( IF 3.9 ) Pub Date : 2021-02-23 , DOI: 10.1021/acs.langmuir.1c00100 Samuel Okyem 1 , Olatunde Awotunde 1 , Tosin Ogunlusi 1 , McKenzie B Riley 1 , Jeremy D Driskell 1
Affiliation
|
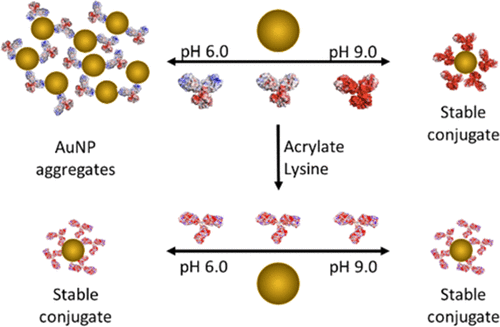
The unique physicochemical properties of gold nanoparticles (AuNPs) provide many opportunities to develop novel biomedical technologies. The surface chemistry of AuNPs can be engineered to perform a variety of functions, including targeted binding, cellular uptake, or stealthlike properties through the immobilization of biomolecules, such as proteins. It is well established that proteins can spontaneously adsorb onto AuNPs, to form a stable and functional bioconjugate; however, the protein–AuNP interaction may result in the formation of less desirable protein–AuNP aggregates. Therefore, it is imperative to investigate the protein–AuNP interaction and elucidate the mechanism by which protein triggers AuNP aggregation. Herein, we systematically investigated the interaction of immunoglobulin G (IgG) antibody with citrate-capped AuNPs as a function of solution pH. We found that the addition of antibody triggers the aggregation of AuNPs for pH < 7.5, whereas a monolayer of antibody adsorbs onto the AuNP to form a stable bioconjugate when the antibody is added to AuNPs at pH ≥ 7.5. Our data identifies electrostatic bridging between the antibody and the negatively charged AuNPs as the mechanism by which aggregation occurs and rules out protein unfolding and surface charge depletion as potential causes. Furthermore, we found that the electrostatic bridging of AuNPs is reversible within the first few hours of interaction, but the protein–AuNP interactions strengthen over 24 h, after which the protein–AuNP aggregate is irreversibly formed. From this data, we developed a straightforward approach to acrylate the basic residues on the antibody to prevent protein-induced aggregation of AuNP over a wide pH range. The results of this study provide additional insight into antibody–nanoparticle interactions and provide a pathway to control the interaction with the potential to enhance the conjugate function.
中文翻译:

探索抗体触发金纳米粒子聚集的机理。
金纳米颗粒(AuNPs)的独特理化性质为开发新型生物医学技术提供了许多机会。可以通过固定化生物分子(例如蛋白质)来设计AuNP的表面化学,以执行多种功能,包括靶向结合,细胞摄取或隐身性质。众所周知,蛋白质可以自发地吸附到AuNPs上,形成稳定而功能强大的生物结合物。然而,蛋白质-AuNP相互作用可能导致形成较不理想的蛋白质-AuNP聚集体。因此,必须研究蛋白质与AuNP的相互作用,并阐明蛋白质触发AuNP聚集的机制。在此处,我们系统地研究了免疫球蛋白G(IgG)抗体与柠檬酸盐封端的AuNP的相互作用,其与溶液pH的关系。我们发现,添加抗体会触发pH <7.5时AuNP的聚集,而当将抗体添加到pH≥7.5的AuNPs中时,单层抗体会吸附到AuNP上以形成稳定的生物结合物。我们的数据确定了抗体与带负电荷的AuNPs之间的静电桥接是发生聚集的机制,并排除了蛋白质解折叠和表面电荷耗竭的潜在原因。此外,我们发现AuNPs的静电桥接在相互作用的最初几个小时内是可逆的,但是蛋白-AuNP的相互作用在24小时内增强,此后蛋白-AuNP的聚集体不可逆地形成。根据这些数据,我们开发了一种简单的方法,对抗体上的基本残基进行丙烯酸酯化,以防止蛋白质在宽pH范围内引起AuNP聚集。这项研究的结果提供了对抗体-纳米颗粒相互作用的更多见解,并提供了一种控制相互作用的途径,并具有增强缀合物功能的潜力。
更新日期:2021-03-09
中文翻译:

探索抗体触发金纳米粒子聚集的机理。
金纳米颗粒(AuNPs)的独特理化性质为开发新型生物医学技术提供了许多机会。可以通过固定化生物分子(例如蛋白质)来设计AuNP的表面化学,以执行多种功能,包括靶向结合,细胞摄取或隐身性质。众所周知,蛋白质可以自发地吸附到AuNPs上,形成稳定而功能强大的生物结合物。然而,蛋白质-AuNP相互作用可能导致形成较不理想的蛋白质-AuNP聚集体。因此,必须研究蛋白质与AuNP的相互作用,并阐明蛋白质触发AuNP聚集的机制。在此处,我们系统地研究了免疫球蛋白G(IgG)抗体与柠檬酸盐封端的AuNP的相互作用,其与溶液pH的关系。我们发现,添加抗体会触发pH <7.5时AuNP的聚集,而当将抗体添加到pH≥7.5的AuNPs中时,单层抗体会吸附到AuNP上以形成稳定的生物结合物。我们的数据确定了抗体与带负电荷的AuNPs之间的静电桥接是发生聚集的机制,并排除了蛋白质解折叠和表面电荷耗竭的潜在原因。此外,我们发现AuNPs的静电桥接在相互作用的最初几个小时内是可逆的,但是蛋白-AuNP的相互作用在24小时内增强,此后蛋白-AuNP的聚集体不可逆地形成。根据这些数据,我们开发了一种简单的方法,对抗体上的基本残基进行丙烯酸酯化,以防止蛋白质在宽pH范围内引起AuNP聚集。这项研究的结果提供了对抗体-纳米颗粒相互作用的更多见解,并提供了一种控制相互作用的途径,并具有增强缀合物功能的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号