当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reactive Species and Reaction Pathways for the Oxidative Cleavage of 4-Octene and Oleic Acid with H2O2 over Tungsten Oxide Catalysts
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-24 , DOI: 10.1021/acscatal.0c05393 Danim Yun 1, 2 , E. Zeynep Ayla 2 , Daniel T. Bregante 2 , David W. Flaherty 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-24 , DOI: 10.1021/acscatal.0c05393 Danim Yun 1, 2 , E. Zeynep Ayla 2 , Daniel T. Bregante 2 , David W. Flaherty 1, 2
Affiliation
|
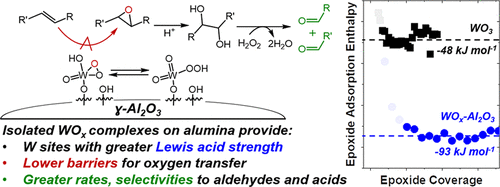
Oxidative cleavage of carbon–carbon double bonds (C═C) in alkenes and fatty acids produces aldehydes and acids valued as chemical intermediates. Solid tungsten oxide catalysts are low cost, nontoxic, and selective for the oxidative cleavage of C═C bonds with hydrogen peroxide (H2O2) and are, therefore, a promising option for continuous processes. Despite the relevance of these materials, the elementary steps involved and their sensitivity to the form of W sites present on surfaces have not been described. Here, we combine in situ spectroscopy and rate measurements to identify significant steps in the reaction and the reactive species present on the catalysts and examine differences between the kinetics of this reaction on isolated W atoms grafted to alumina and on those exposed on crystalline WO3 nanoparticles. Raman spectroscopy shows that W–peroxo complexes (W–(η2-O2)) formed from H2O2 react with alkenes in a kinetically relevant step to produce epoxides, which undergo hydrolysis at protic surface sites. Subsequently, the CH3CN solvent deprotonates diols to form alpha-hydroxy ketones that react to form aldehydes and water following nucleophilic attack of H2O2. Turnover rates for oxidative cleavage, determined by in situ site titrations, on WOx–Al2O3 are 75% greater than those on WO3 at standard conditions. These differences reflect the activation enthalpies (ΔH‡) for the oxidative cleavage of 4-octene that are much lower than those for the isolated WOx sites (36 ± 3 and 60 ± 6 kJ·mol–1 for WOx–Al2O3 and WO3, respectively) and correlate strongly with the difference between the enthalpies of adsorption for epoxyoctane (ΔHads,epox), which resembles the transition state for epoxidation. The WOx–Al2O3 catalysts mediate oxidative cleavage of oleic acid with H2O2 following a mechanism comparable to that for the oxidative cleavage of 4-octene. The WO3 materials, however, form only the epoxide and do not cleave the C–C bond or produce aldehydes and acids. These differences reflect the distinct site requirements for these reaction pathways and indicate that acid sites required for diol formation are strongly inhibited by oleic acids and epoxides on WO3 whereas the Al2O3 support provides sites competent for this reaction and increase the yield of the oxidative cleavage products.
中文翻译:

氧化钨催化剂上H 2 O 2对4-辛烯和油酸与H 2 O 2的氧化裂解的反应物种和反应途径
烯烃和脂肪酸中碳-碳双键(C═C)的氧化裂解会产生醛和酸,它们被视作化学中间体。固态氧化钨催化剂价格低廉,无毒且对用过氧化氢(H 2 O 2),因此是连续过程的理想选择。尽管这些材料相关,但尚未描述所涉及的基本步骤及其对表面上W位置形式的敏感性。在这里,我们结合原位光谱和速率测量来确定反应中的重要步骤和催化剂上存在的反应性物种,并检查该反应动力学对接枝到氧化铝上的孤立W原子和在结晶WO 3纳米颗粒上暴露的那些动力学之间的差异。。拉曼光谱显示,W-过氧复合物(W-(η 2 -O 2))选自H形成2 ö 2在动力学上相关的步骤中与烯烃反应生成环氧化物,该环氧化物在质子表面位点发生水解。随后,CH 3 CN溶剂使二醇去质子化以形成α-羟基酮,在H 2 O 2发生亲核攻击后,后者反应形成醛和水。在标准条件下,WO x -Al 2 O 3上通过原位滴定法测定的氧化裂解的转换速率比WO 3的转换速率高75%。这些差异反映了4-辛烯的氧化裂解的活化焓(ΔH ‡)远低于分离的WO x的活化焓(ΔH ‡)。位点(36±3和60±6千焦·摩尔-1为WO X -Al 2 ö 3和WO 3,分别地),并用吸附的对环氧辛烷焓(之间的差相关成分强烈ΔH广告,磐英),它类似于环氧化的过渡态。WO x -Al 2 O 3催化剂以与4-辛烯氧化裂解相当的机理介导油酸与H 2 O 2的氧化裂解。WO 3但是,这些材料仅形成环氧化物,不会裂解C–C键或产生醛和酸。这些差异反映了这些反应途径的不同位点要求,并表明二醇形成所需的酸位点受到WO 3上的油酸和环氧化物的强烈抑制,而Al 2 O 3载体则提供了适合该反应的位点并增加了反应的收率。氧化裂解产物。
更新日期:2021-03-05
中文翻译:

氧化钨催化剂上H 2 O 2对4-辛烯和油酸与H 2 O 2的氧化裂解的反应物种和反应途径
烯烃和脂肪酸中碳-碳双键(C═C)的氧化裂解会产生醛和酸,它们被视作化学中间体。固态氧化钨催化剂价格低廉,无毒且对用过氧化氢(H 2 O 2),因此是连续过程的理想选择。尽管这些材料相关,但尚未描述所涉及的基本步骤及其对表面上W位置形式的敏感性。在这里,我们结合原位光谱和速率测量来确定反应中的重要步骤和催化剂上存在的反应性物种,并检查该反应动力学对接枝到氧化铝上的孤立W原子和在结晶WO 3纳米颗粒上暴露的那些动力学之间的差异。。拉曼光谱显示,W-过氧复合物(W-(η 2 -O 2))选自H形成2 ö 2在动力学上相关的步骤中与烯烃反应生成环氧化物,该环氧化物在质子表面位点发生水解。随后,CH 3 CN溶剂使二醇去质子化以形成α-羟基酮,在H 2 O 2发生亲核攻击后,后者反应形成醛和水。在标准条件下,WO x -Al 2 O 3上通过原位滴定法测定的氧化裂解的转换速率比WO 3的转换速率高75%。这些差异反映了4-辛烯的氧化裂解的活化焓(ΔH ‡)远低于分离的WO x的活化焓(ΔH ‡)。位点(36±3和60±6千焦·摩尔-1为WO X -Al 2 ö 3和WO 3,分别地),并用吸附的对环氧辛烷焓(之间的差相关成分强烈ΔH广告,磐英),它类似于环氧化的过渡态。WO x -Al 2 O 3催化剂以与4-辛烯氧化裂解相当的机理介导油酸与H 2 O 2的氧化裂解。WO 3但是,这些材料仅形成环氧化物,不会裂解C–C键或产生醛和酸。这些差异反映了这些反应途径的不同位点要求,并表明二醇形成所需的酸位点受到WO 3上的油酸和环氧化物的强烈抑制,而Al 2 O 3载体则提供了适合该反应的位点并增加了反应的收率。氧化裂解产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号