当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanisms of Hydrogen Evolution Reaction in Two-Dimensional Nitride MXenes Using In Situ X-Ray Absorption Spectroelectrochemistry
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-24 , DOI: 10.1021/acscatal.0c05634 Abdoulaye Djire 1, 2 , Hanyu Zhang 1 , Benjamin J. Reinhart 3 , O. Charles Nwamba 1 , Nathan R. Neale 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-24 , DOI: 10.1021/acscatal.0c05634 Abdoulaye Djire 1, 2 , Hanyu Zhang 1 , Benjamin J. Reinhart 3 , O. Charles Nwamba 1 , Nathan R. Neale 1
Affiliation
|
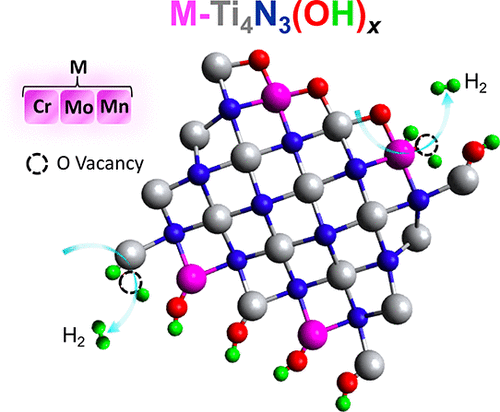
Two-dimensional (2D) MXenes based on early transition-metal carbides and nitrides have highly tunable electrochemical properties including excellent catalytic activity for the hydrogen evolution reaction (HER). Compared to carbide MXenes, nitride MXenes feature metal–nitrogen bonds that are expected to impart unique electronic and structural characteristics for electrocatalysis. Recently, we showed that the HER activity can be enhanced by modifying a pristine exfoliated Ti4N3Tx MXene with various metal ions to produce mixed-metal nitride MXenes M-Ti4N3Tx (M = V, Cr, Mo, and Mn; Tx = O and/or OH). Here, we use in situ X-ray absorption spectroscopy (XAS) to elucidate the mechanisms of HER activity in these 2D M-Ti4N3Tx MXenes. X-ray absorption near-edge structure (XANES) results confirm the presence of multiple oxidation states in the Ti4N3Tx and M-Ti4N3Tx MXenes during electrochemical and HER activities. In most cases, metal-ion oxidation states are unaffected over the potential range studied except for the Cr-Ti4N3Tx MXene that undergoes a partial reduction of Cr3+ to Cr2+ during HER. The structural analysis of the M-Ti4N3Tx MXenes under electrochemical and HER conditions is provided by the extended X-ray absorption fine structure (EXAFS), which further reveals that the mechanism of HER catalysis involves the creation of oxygen vacancies on the basal planes. The XAS data are clear that these oxygen vacancies are coincident with metal-ion reduction, which combined generate active sites for HER. We find that the alloyed metal can either change the reduction potential of the Ti4+ ions (in the Mo-Ti4N3Tx MXene) or act as a single-atom catalyst (in the Cr-Ti4N3Tx MXene), both of which can be exploited to tune the activity in electrocatalysis and photocatalysis schemes.
中文翻译:

原位X射线吸收光谱电化学分析二维氮化物MXene中的氢生成反应机理
基于早期过渡金属碳化物和氮化物的二维(2D)MXene具有高度可调节的电化学特性,包括对氢释放反应(HER)的出色催化活性。与碳化物MXene相比,氮化物MXene具有金属-氮键,有望为电催化赋予独特的电子和结构特征。最近,我们表明,可以通过用各种金属离子修饰原始剥离的Ti 4 N 3 T x MXene来生产混合金属氮化物MXene M-Ti 4 N 3 T x(M = V,Cr,Mo等)来增强HER活性。,和Mn; T x= O和/或OH)。在这里,我们使用原位X射线吸收光谱(XAS)来阐明这些2D M-Ti 4 N 3 T x MXenes中HER活性的机制。X射线吸收近边缘结构(XANES)结果证实,在电化学和HER活性期间,Ti 4 N 3 T x和M-Ti 4 N 3 T x MXene中存在多个氧化态。在大多数情况下,除了Cr-Ti 4 N 3 T x MXene会部分还原Cr 3+到Cr之外,在研究的电位范围内金属离子的氧化态不会受到影响。HER期间为2+。扩展的X射线吸收精细结构(EXAFS)提供了在电化学和HER条件下M-Ti 4 N 3 T x MXenes的结构分析,这进一步揭示了HER催化的机理涉及在C上形成氧空位。基面。XAS数据清楚地表明,这些氧空位与金属离子还原同时发生,金属离子还原共同产生了HER的活性位点。我们发现合金化金属可以改变Ti 4+离子的还原电势(在Mo-Ti 4 N 3 T x MXene中)或充当单原子催化剂(在Cr-Ti 4 N 3 T中)x MXene),两者均可用于调节电催化和光催化方案中的活性。
更新日期:2021-03-05
中文翻译:

原位X射线吸收光谱电化学分析二维氮化物MXene中的氢生成反应机理
基于早期过渡金属碳化物和氮化物的二维(2D)MXene具有高度可调节的电化学特性,包括对氢释放反应(HER)的出色催化活性。与碳化物MXene相比,氮化物MXene具有金属-氮键,有望为电催化赋予独特的电子和结构特征。最近,我们表明,可以通过用各种金属离子修饰原始剥离的Ti 4 N 3 T x MXene来生产混合金属氮化物MXene M-Ti 4 N 3 T x(M = V,Cr,Mo等)来增强HER活性。,和Mn; T x= O和/或OH)。在这里,我们使用原位X射线吸收光谱(XAS)来阐明这些2D M-Ti 4 N 3 T x MXenes中HER活性的机制。X射线吸收近边缘结构(XANES)结果证实,在电化学和HER活性期间,Ti 4 N 3 T x和M-Ti 4 N 3 T x MXene中存在多个氧化态。在大多数情况下,除了Cr-Ti 4 N 3 T x MXene会部分还原Cr 3+到Cr之外,在研究的电位范围内金属离子的氧化态不会受到影响。HER期间为2+。扩展的X射线吸收精细结构(EXAFS)提供了在电化学和HER条件下M-Ti 4 N 3 T x MXenes的结构分析,这进一步揭示了HER催化的机理涉及在C上形成氧空位。基面。XAS数据清楚地表明,这些氧空位与金属离子还原同时发生,金属离子还原共同产生了HER的活性位点。我们发现合金化金属可以改变Ti 4+离子的还原电势(在Mo-Ti 4 N 3 T x MXene中)或充当单原子催化剂(在Cr-Ti 4 N 3 T中)x MXene),两者均可用于调节电催化和光催化方案中的活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号