当前位置:
X-MOL 学术
›
Brain Pathol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accumulation of cellular prion protein within β-amyloid oligomer plaques in aged human brains
Brain Pathology ( IF 6.4 ) Pub Date : 2021-02-23 , DOI: 10.1111/bpa.12941 Reisuke H Takahashi 1, 2 , Mayumi Yokotsuka 1 , Minoru Tobiume 2 , Yuko Sato 2 , Hideki Hasegawa 2 , Toshitaka Nagao 1 , Gunnar K Gouras 3
Brain Pathology ( IF 6.4 ) Pub Date : 2021-02-23 , DOI: 10.1111/bpa.12941 Reisuke H Takahashi 1, 2 , Mayumi Yokotsuka 1 , Minoru Tobiume 2 , Yuko Sato 2 , Hideki Hasegawa 2 , Toshitaka Nagao 1 , Gunnar K Gouras 3
Affiliation
|
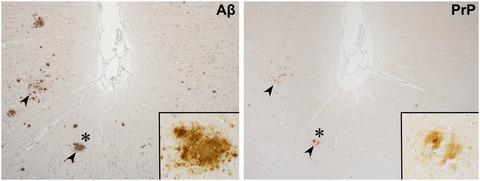
Alzheimer’s disease (AD) is the main cause of dementia, and β-amyloid (Aβ) is a central factor in the initiation and progression of the disease. Different forms of Aβ have been identified as monomers, oligomers, and amyloid fibrils. Many proteins have been implicated as putative receptors of respective forms of Aβ. Distinct forms of Aβ oligomers are considered to be neurotoxic species that trigger the pathophysiology of AD. It was reported that cellular prion protein (PrPC) is one of the most selective and high-affinity binding partners of Aβ oligomers. The interaction of Aβ oligomers with PrPC is important to synaptic dysfunction and loss. The binding of Aβ oligomers to PrPC has mostly been studied with synthetic peptides, cell culture, and murine models of AD by biochemical and biological methods. However, the molecular mechanisms underlying the relationship between Aβ oligomers and PrPC remain unclear, especially in the human brain. We immunohistochemically investigated the relationship between Aβ oligomers and PrPC in human brain tissue with and without amyloid pathology. We histologically demonstrate that PrPC accumulates with aging in human brain tissue even prior to AD mainly within diffuse-type amyloid plaques, which are composed of more soluble Aβ oligomers without stacked β-sheet fibril structures. Our results suggest that PrPC accumulating plaques are associated with more soluble Aβ oligomers, and appear even prior to AD. The investigation of PrPC accumulating plaques may provide new insights into AD.
中文翻译:

老年人大脑中β-淀粉样蛋白寡聚体斑块中细胞朊病毒蛋白的积累
阿尔茨海默病(AD)是痴呆的主要原因,而β-淀粉样蛋白(Aβ)是该疾病发生和发展的核心因素。不同形式的 Aβ 已被确定为单体、寡聚体和淀粉样蛋白原纤维。许多蛋白质被认为是各种 Aβ 形式的假定受体。不同形式的 Aβ 寡聚体被认为是引发 AD 病理生理学的神经毒性物质。据报道,细胞朊病毒蛋白 (PrP C ) 是 Aβ 寡聚体最具选择性和高亲和力的结合伙伴之一。Aβ 寡聚体与 PrP C的相互作用对突触功能障碍和丧失很重要。Aβ寡聚体与PrP C的结合主要通过生化和生物学方法用合成肽、细胞培养和 AD 小鼠模型进行研究。然而,Aβ寡聚体与PrP C之间关系的分子机制仍不清楚,尤其是在人脑中。我们免疫组织化学研究了有和没有淀粉样蛋白病理学的人脑组织中Aβ 寡聚体和 PrP C之间的关系。我们在组织学上证明,即使在 AD 之前,PrP C在人脑组织中随着衰老而积累,主要是在弥漫型淀粉样斑块中,这些斑块由更可溶的 Aβ 寡聚体组成,没有堆叠的 β-折叠原纤维结构。我们的结果表明,PrP C积聚的斑块与更易溶解的 Aβ 寡聚体有关,甚至在 AD 之前就出现了。对 PrP C积聚斑块的研究可能为 AD 提供新的见解。
更新日期:2021-02-23
中文翻译:

老年人大脑中β-淀粉样蛋白寡聚体斑块中细胞朊病毒蛋白的积累
阿尔茨海默病(AD)是痴呆的主要原因,而β-淀粉样蛋白(Aβ)是该疾病发生和发展的核心因素。不同形式的 Aβ 已被确定为单体、寡聚体和淀粉样蛋白原纤维。许多蛋白质被认为是各种 Aβ 形式的假定受体。不同形式的 Aβ 寡聚体被认为是引发 AD 病理生理学的神经毒性物质。据报道,细胞朊病毒蛋白 (PrP C ) 是 Aβ 寡聚体最具选择性和高亲和力的结合伙伴之一。Aβ 寡聚体与 PrP C的相互作用对突触功能障碍和丧失很重要。Aβ寡聚体与PrP C的结合主要通过生化和生物学方法用合成肽、细胞培养和 AD 小鼠模型进行研究。然而,Aβ寡聚体与PrP C之间关系的分子机制仍不清楚,尤其是在人脑中。我们免疫组织化学研究了有和没有淀粉样蛋白病理学的人脑组织中Aβ 寡聚体和 PrP C之间的关系。我们在组织学上证明,即使在 AD 之前,PrP C在人脑组织中随着衰老而积累,主要是在弥漫型淀粉样斑块中,这些斑块由更可溶的 Aβ 寡聚体组成,没有堆叠的 β-折叠原纤维结构。我们的结果表明,PrP C积聚的斑块与更易溶解的 Aβ 寡聚体有关,甚至在 AD 之前就出现了。对 PrP C积聚斑块的研究可能为 AD 提供新的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号