Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.4 ) Pub Date : 2021-02-24 , DOI: 10.1016/j.saa.2021.119600 Nicoleta Sandu , Claudia G. Chilom , Aurel I. Popescu
|
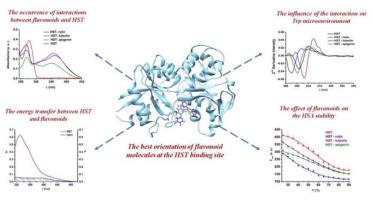
Human serum transferrin (HST) acts as a carrier for Fe3+ and other ions. Binding of flavonoids to HST produces changes in the protein structure with direct implication on iron delivery into cells. We investigate the binding mechanism and affinity towards HST of three flavonoids: rutin, luteolin, and apigenin by different techniques: UV–Vis, fluorescence, fluorescence resonance energy transfer (FRET) combined with molecular docking. UV–Vis results indicate an interaction between flavonoids and HST. It was observed that HST fluorescence was quenched by these three flavonoids via a static process. All the interactions were moderate and the main driving forces are hydrophobic (ΔH > 0 and ΔS > 0) for rutin and luteolin binding or electrostatic (ΔH < 0 and ΔS > 0) for apigenin binding. FRET and molecular docking studies confirm the fluorescence static quenching mechanism by flavonoid binding. The binding of all three flavonoids increases HST stability. These results present the potential use of HST in target-oriented delivery of flavonoids and possibly other drugs into cells.
中文翻译:

类黄酮作为血清转铁蛋白配体的结构和分子方面
人血清转铁蛋白(HST)充当Fe 3+和其他离子的载体。类黄酮与HST的结合产生蛋白质结构的变化,直接影响铁向细胞中的传递。我们通过不同的技术研究了三种类黄酮(芦丁,木犀草素和芹菜素)的结合机制和对HST的亲和力:UV-Vis,荧光,荧光共振能量转移(FRET)与分子对接。UV-Vis结果表明类黄酮与HST之间存在相互作用。观察到,HST荧光通过静态过程被这三种类黄酮淬灭。所有的相互作用都是中等的, 芦丁和木犀草素结合的主要驱动力是疏水性的(ΔH > 0和ΔS > 0)或静电作用(ΔH <0且ΔS > 0)进行芹菜素结合。FRET和分子对接研究证实了类黄酮结合的荧光静态猝灭机制。所有三种类黄酮的结合均增加了HST的稳定性。这些结果表明,HST在将类黄酮和其他可能的药物定向靶向递送至细胞中具有潜在的用途。


























 京公网安备 11010802027423号
京公网安备 11010802027423号