Journal of Advanced Research ( IF 10.7 ) Pub Date : 2021-02-24 , DOI: 10.1016/j.jare.2021.02.004 Hanitrarimalala Veroniaina 1 , Zhenghong Wu 1 , Xiaole Qi 1
|
Introduction
Hypoxic tumor microenvironment (TME) is the major contributor to cancer metastasis, resistance to chemotherapy, and recurrence of tumors. So far, no approved treatment has been available to overcome tumor hypoxia.
Objectives
The present study aimed to relieve tumor hypoxia via a nanozyme theranostic nanomaterial as well as providing magnetic resonance imaging (MRI)-guided therapy.
Methods
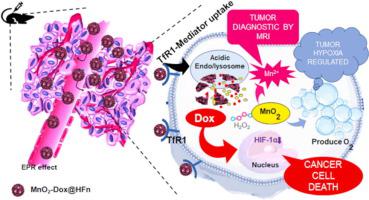
Manganese dioxide (MnO2) was used for its intrinsic enzymatic activity co-loaded with the anti-cancer drug Doxorubicin (Dox) within the recombinant heavy-chain apoferritin cavity to form MnO2-Dox@HFn. Following the synthesis of the nanomaterial, different characterizations were performed as well as its nanozyme-like ability. This nanoplatform recognizes tumor cells through the transferrin receptors 1 (TfR1) which are highly expressed on the surface of most cancer cells. The cellular uptake was confirmed by flow cytometry and fluorescence spectroscopy. In vitro and in vivo studies have been investigated to evaluate the hypoxia regulation, MRI ability and anti-tumor activity of MnO2-Dox@HFn.
Results
Being a TME-responsive nanomaterial, MnO2-Dox@HFn exerted both peroxidase and catalase activity that mainly produce massive oxygen and Mn2+ ions. Respectively, these products relieve the unfavorable tumor hypoxia and also exhibit T1-weighted MRI with a high longitudinal relaxivity of 33.40 mM. s−1. The utility of MnO2-Dox@HFn was broadened with their efficient anti-cancer activity proved both in vitro and in vivo.
Conclusions
MnO2-Dox@HFn successfully overcome tumor hypoxia with double potentials enzymatic ability and diagnostic capacity. This investigation could ignite the future application for cancer theranostic nanozyme therapy.
中文翻译:

先天性肿瘤靶向纳米酶克服肿瘤缺氧用于癌症治疗
介绍
缺氧肿瘤微环境(TME)是导致癌症转移、化疗耐药和肿瘤复发的主要因素。到目前为止,还没有获得批准的治疗方法来克服肿瘤缺氧。
目标
本研究旨在通过纳米酶治疗纳米材料缓解肿瘤缺氧,并提供磁共振成像 (MRI) 引导的治疗。
方法
二氧化锰 (MnO 2 ) 因其内在的酶活性与重组重链脱铁铁蛋白空腔内的抗癌药物阿霉素 (Dox) 共同负载形成 MnO 2 -Dox@HFn。在合成纳米材料之后,进行了不同的表征以及其类似纳米酶的能力。该纳米平台通过在大多数癌细胞表面高度表达的转铁蛋白受体 1 (TfR1) 识别肿瘤细胞。通过流式细胞术和荧光光谱证实细胞摄取。已经研究了体外和体内研究以评估MnO 2 -Dox@HFn的缺氧调节、MRI能力和抗肿瘤活性。
结果
MnO 2 -Dox@HFn作为一种 TME 响应纳米材料,同时具有过氧化物酶和过氧化氢酶活性,主要产生大量的氧和 Mn 2+离子。这些产品分别缓解了不利的肿瘤缺氧,并表现出 T1 加权 MRI 具有 33.40 mM 的高纵向弛豫度。s -1。MnO 2 -Dox@HFn 的用途得到了扩展,其有效的抗癌活性在体外和体内都得到了证实。
结论
MnO 2 -Dox@HFn 以双电位酶促能力和诊断能力成功克服肿瘤缺氧。这项研究可能会点燃癌症治疗纳米酶治疗的未来应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号