当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical reduction of CO2 towards multi-carbon products via a two-step process
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2021-2-5 , DOI: 10.1039/d1re00001b Xianbiao Fu 1, 2, 3, 4 , Jiahao Zhang 1, 2, 3, 4 , Yijin Kang 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2021-2-5 , DOI: 10.1039/d1re00001b Xianbiao Fu 1, 2, 3, 4 , Jiahao Zhang 1, 2, 3, 4 , Yijin Kang 1, 2, 3, 4
Affiliation
|
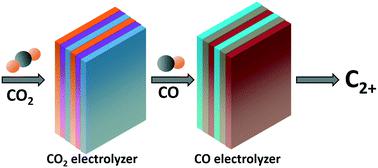
The electrochemical conversion of carbon dioxide (CO2) towards clean fuels and chemicals powered by renewable energy is a promising strategy to realize the closing of the loop of carbon footprint. However, the direct reduction of CO2 to multi-carbon (C2+) products suffers from low activity in non-alkaline electrolyte or electrolyte degradation problem caused by carbonate formation in alkaline electrolyte. The two-step process for CO2 electrocution can circumvent such problems by converting CO2 to CO (the first step) in the non-alkaline electrolyte and promote the rate of carbon–carbon coupling for CO-to-C2+ conversion (the second step) in alkaline electrolytes. We summarize the recent progress of CO-selective catalysts, C2+-selective catalysts, tandem catalysts, and tandem reaction systems, which aim to achieve the efficient production of C2+ products with high selectivity. The two-step route of CO2 reduction pushes the chemical production from environmentally abundant molecules closer to the practical application, offering a promising replacement in the petrochemical industry for chemical production under hydrogen economy in the future.
中文翻译:

通过两步法电化学将二氧化碳还原为多碳产品
将二氧化碳(CO 2)电化学转化为由可再生能源提供动力的清洁燃料和化学物质,是实现封闭碳足迹回路的一种有前途的策略。然而,将CO 2直接还原成多碳(C 2+)产物的缺点是在非碱性电解质中的活性低或碱性电解质中碳酸盐形成引起的电解质降解问题。通过在非碱性电解液中将CO 2转化为CO(第一步),分两步进行CO 2电死的过程可以避免此类问题,并提高CO-to-C 2+的碳-碳偶联速率在碱性电解液中的转化(第二步)。我们总结了CO选择催化剂,C 2+选择催化剂,串联催化剂和串联反应体系的最新进展,其目的是实现高效生产高选择性的C 2+产物。减少CO 2的两步路线使来自环境丰富分子的化学生产更接近于实际应用,为石油化工行业未来氢经济下的化学生产提供了有希望的替代方法。
更新日期:2021-02-23
中文翻译:

通过两步法电化学将二氧化碳还原为多碳产品
将二氧化碳(CO 2)电化学转化为由可再生能源提供动力的清洁燃料和化学物质,是实现封闭碳足迹回路的一种有前途的策略。然而,将CO 2直接还原成多碳(C 2+)产物的缺点是在非碱性电解质中的活性低或碱性电解质中碳酸盐形成引起的电解质降解问题。通过在非碱性电解液中将CO 2转化为CO(第一步),分两步进行CO 2电死的过程可以避免此类问题,并提高CO-to-C 2+的碳-碳偶联速率在碱性电解液中的转化(第二步)。我们总结了CO选择催化剂,C 2+选择催化剂,串联催化剂和串联反应体系的最新进展,其目的是实现高效生产高选择性的C 2+产物。减少CO 2的两步路线使来自环境丰富分子的化学生产更接近于实际应用,为石油化工行业未来氢经济下的化学生产提供了有希望的替代方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号