当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of Anion-Catalyzed C–H Silylation Using TMSCF3: Kinetically-Controlled CF3-Anionoid Partitioning As a Key Parameter
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-22 , DOI: 10.1021/acscatal.1c00033 Andrés García-Domínguez 1 , Pedro H. Helou de Oliveira 1 , Gilian T. Thomas 1 , Arnau R. Sugranyes 1 , Guy C. Lloyd-Jones 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-02-22 , DOI: 10.1021/acscatal.1c00033 Andrés García-Domínguez 1 , Pedro H. Helou de Oliveira 1 , Gilian T. Thomas 1 , Arnau R. Sugranyes 1 , Guy C. Lloyd-Jones 1
Affiliation
|
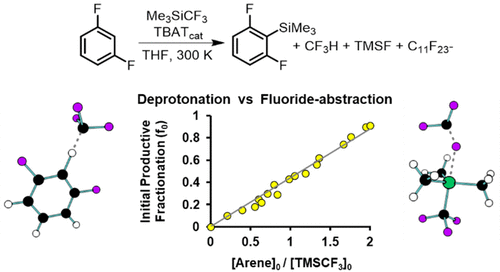
The mechanism of anion-catalyzed C–H silylation by R3SiCF3 reagents has been investigated using homogeneous TBAT-initiation, in situ and stopped-flow 19F NMR spectroscopy 2H-KIE, LFER, deuterium-labeled crossover, structure-selectivity quantitation (TMSCF3/TESCF3), carbene trapping, and DFT-calculations. Analysis of the kinetics of reactions of 1,3-difluorobenzenes (2), and the generation of ArSiMe3 and Me3SiF as a function of the concentration of [2], [TMSCF3], and [TBAT], show that a CF3-anionoid is the active intermediate. The CF3-anionoid is reversibly released from siliconate [(CF3)2SiMe3]− and undergoes partitioning through rate-limiting arene deprotonation (1H/2H KIE 9.5) to generate ArSiMe3 (via a transient aryl anionoid) and fluoroform (CF3H), in competition with F-anion transfer to TMSCF3 to generate CF2 and TMSF. The [2]/[TMSCF3] concentration ratio directly and proportionally controls the kinetics of the partition, in favor of C–H deprotonation. Higher concentrations of TBAT and lower concentrations of TMSCF3 lead to faster rates of ArSiMe3 generation. Use of the homologous TESCF3 reagent leads to faster rates of anion catalysis and an increased selectivity toward C–H deprotonation. Perfluoroalkenes, generated in situ from CF2, capture the CF3-anionoid leading to progressive inhibition of the anion-catalysis. Inhibition is suppressed by using a styrene additive to trap the CF2 and the efficiency of the process enhanced by slow-addition of TMSCF3 (1) to maintain a high concentration ratio [2]/[1].
中文翻译:

使用TMSCF 3的阴离子催化C–H硅烷化的机理:以动力学控制的CF 3-阴离子分配为关键参数
使用均相的TBAT引发,原位和停止流19 F NMR光谱2 H-KIE,LFER,氘标记的交叉,结构选择性,研究了R 3 SiCF 3试剂对阴离子催化的CHH甲硅烷基化的机理。定量分析(TMSCF 3 / TESCF 3),卡宾捕获和DFT计算。分析1,3-二氟苯(2)的反应动力学,以及ArSiMe 3和Me 3 SiF的生成与[ 2 ],[TMSCF 3 ]和[TBAT]浓度的关系,表明CF 3-阴离子型是活性中间体。CF 3-阴离子可逆地从硅酸盐[(CF 3)2 SiMe 3 ] -中释放出来,并通过限速芳烃去质子化反应(1 H / 2 H KIE 9.5)进行分配,生成ArSiMe 3(通过瞬态芳基阴离子)。氟仿(CF 3 H),与F阴离子转移到TMSCF 3中竞争,生成CF 2和TMSF。[ 2 ] / [TMSCF 3]浓度比可直接和成比例地控制隔板的动力学,有利于C–H去质子化。较高浓度的TBAT和较低浓度的TMSCF 3导致ArSiMe 3生成速度更快。使用同源TESCF 3试剂可加快阴离子催化速率,并增加对CH去质子化的选择性。从CF 2原位生成的全氟烯烃捕获CF 3-阴离子,导致阴离子催化的逐步抑制。通过使用苯乙烯添加剂捕获CF 2可以抑制抑制作用,并且通过缓慢添加TMSCF 3(1可以提高该过程的效率)以保持高浓度比[ 2 ] / [ 1 ]。
更新日期:2021-03-05
中文翻译:

使用TMSCF 3的阴离子催化C–H硅烷化的机理:以动力学控制的CF 3-阴离子分配为关键参数
使用均相的TBAT引发,原位和停止流19 F NMR光谱2 H-KIE,LFER,氘标记的交叉,结构选择性,研究了R 3 SiCF 3试剂对阴离子催化的CHH甲硅烷基化的机理。定量分析(TMSCF 3 / TESCF 3),卡宾捕获和DFT计算。分析1,3-二氟苯(2)的反应动力学,以及ArSiMe 3和Me 3 SiF的生成与[ 2 ],[TMSCF 3 ]和[TBAT]浓度的关系,表明CF 3-阴离子型是活性中间体。CF 3-阴离子可逆地从硅酸盐[(CF 3)2 SiMe 3 ] -中释放出来,并通过限速芳烃去质子化反应(1 H / 2 H KIE 9.5)进行分配,生成ArSiMe 3(通过瞬态芳基阴离子)。氟仿(CF 3 H),与F阴离子转移到TMSCF 3中竞争,生成CF 2和TMSF。[ 2 ] / [TMSCF 3]浓度比可直接和成比例地控制隔板的动力学,有利于C–H去质子化。较高浓度的TBAT和较低浓度的TMSCF 3导致ArSiMe 3生成速度更快。使用同源TESCF 3试剂可加快阴离子催化速率,并增加对CH去质子化的选择性。从CF 2原位生成的全氟烯烃捕获CF 3-阴离子,导致阴离子催化的逐步抑制。通过使用苯乙烯添加剂捕获CF 2可以抑制抑制作用,并且通过缓慢添加TMSCF 3(1可以提高该过程的效率)以保持高浓度比[ 2 ] / [ 1 ]。



























 京公网安备 11010802027423号
京公网安备 11010802027423号