当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sleuthing biochemical evidence to elucidate unassigned electron density in a CBL–SLAP2 crystal complex
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2021-02-23 , DOI: 10.1107/s2053230x21000911 Leanne E Wybenga-Groot 1 , C Jane McGlade 2
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2021-02-23 , DOI: 10.1107/s2053230x21000911 Leanne E Wybenga-Groot 1 , C Jane McGlade 2
Affiliation
|
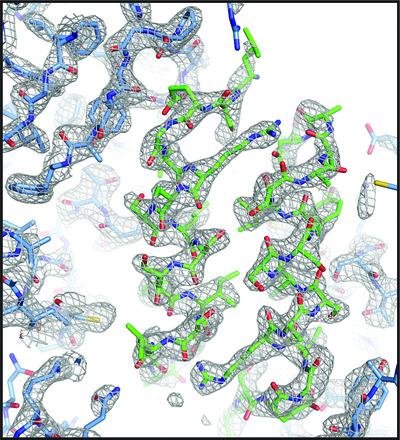
The Src‐like adaptor proteins (SLAP/SLAP2) bind to CBL E3 ubiquitin ligase to downregulate antigen, cytokine and tyrosine kinase receptor signalling. In contrast to the phosphotyrosine‐dependent binding of CBL substrates through its tyrosine kinase‐binding domain (TKBD), CBL TKBD associates with the C‐terminal tail of SLAP2 in a phospho‐independent manner. To understand the distinct nature of this interaction, a purification protocol for SLAP2 in complex with CBL TKBD was established and the complex was crystallized. However, determination of the complex crystal structure was hindered by the apparent degradation of SLAP2 during the crystallization process, such that only the CBL TKBD residues could initially be modelled. Close examination of the CBL TKBD structure revealed a unique dimer interface that included two short segments of electron density of unknown origin. To elucidate which residues of SLAP2 to model into this unassigned density, a co‐expression system was generated to test SLAP2 deletion mutants and define the minimal SLAP2 binding region. SLAP2 degradation products were also analysed by mass spectrometry. The model‐building and map‐generation features of the Phenix software package were employed, leading to successful modelling of the C‐terminal tail of SLAP2 into the unassigned electron‐density segments.
中文翻译:

侦查生化证据以阐明 CBL-SLAP2 晶体复合物中未指定的电子密度
Src 样衔接蛋白 (SLAP/SLAP2) 与 CBL E3 泛素连接酶结合以下调抗原、细胞因子和酪氨酸激酶受体信号传导。与 CBL 底物通过其酪氨酸激酶结合结构域 (TKBD) 的磷酸化酪氨酸结合不同,CBL TKBD 以非磷酸化方式与 SLAP2 的 C 末端尾部结合。为了了解这种相互作用的独特性质,建立了 SLAP2 与 CBL TKBD 复合物的纯化方案,并使复合物结晶。然而,复杂晶体结构的确定受到结晶过程中 SLAP2 明显降解的阻碍,因此最初只能模拟 CBL TKBD 残基。仔细检查 CBL TKBD 结构揭示了一个独特的二聚体界面,其中包括两个未知来源的电子密度短片段。为了阐明 SLAP2 的哪些残基可以模拟到这种未分配的密度,我们生成了一个共表达系统来测试 SLAP2 缺失突变体并定义最小的 SLAP2 结合区域。SLAP2 降解产物也通过质谱分析。模型构建和地图生成功能使用了Phenix软件包,成功地将 SLAP2 的 C 末端尾部建模为未分配的电子密度段。
更新日期:2021-02-23
中文翻译:

侦查生化证据以阐明 CBL-SLAP2 晶体复合物中未指定的电子密度
Src 样衔接蛋白 (SLAP/SLAP2) 与 CBL E3 泛素连接酶结合以下调抗原、细胞因子和酪氨酸激酶受体信号传导。与 CBL 底物通过其酪氨酸激酶结合结构域 (TKBD) 的磷酸化酪氨酸结合不同,CBL TKBD 以非磷酸化方式与 SLAP2 的 C 末端尾部结合。为了了解这种相互作用的独特性质,建立了 SLAP2 与 CBL TKBD 复合物的纯化方案,并使复合物结晶。然而,复杂晶体结构的确定受到结晶过程中 SLAP2 明显降解的阻碍,因此最初只能模拟 CBL TKBD 残基。仔细检查 CBL TKBD 结构揭示了一个独特的二聚体界面,其中包括两个未知来源的电子密度短片段。为了阐明 SLAP2 的哪些残基可以模拟到这种未分配的密度,我们生成了一个共表达系统来测试 SLAP2 缺失突变体并定义最小的 SLAP2 结合区域。SLAP2 降解产物也通过质谱分析。模型构建和地图生成功能使用了Phenix软件包,成功地将 SLAP2 的 C 末端尾部建模为未分配的电子密度段。



























 京公网安备 11010802027423号
京公网安备 11010802027423号