当前位置:
X-MOL 学术
›
Photochem. Photobiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoactive Platinum(II) Azopyridine Complexes†
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2021-02-22 , DOI: 10.1111/php.13405 Sarah J Farley 1 , Luca Salassa 1, 2, 3, 4 , Ana M Pizarro 1, 5 , Peter J Sadler 1
Photochemistry and Photobiology ( IF 3.3 ) Pub Date : 2021-02-22 , DOI: 10.1111/php.13405 Sarah J Farley 1 , Luca Salassa 1, 2, 3, 4 , Ana M Pizarro 1, 5 , Peter J Sadler 1
Affiliation
|
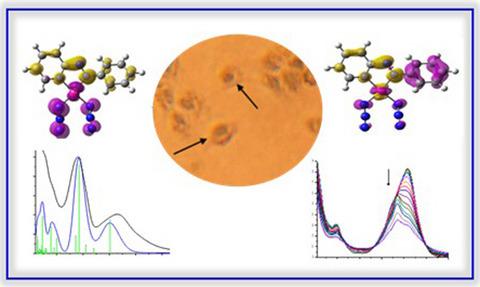
Platinum(II) complexes containing the strong π-acceptor N,N-chelating ligand phenylazopyridine (Ph-azpy) [Pt(p-R-Ph-azpy)X2], R = H, NMe2 or OH, X = Cl or N3, have been synthesized and characterized to explore the effects of monodentate ligands and phenyl substituents on their absorption spectra and photoactivation. Time-dependent density functional theory calculations showed that the complexes have a low-lying unoccupied orbital with strong σ-antibonding character toward the majority of the coordination bonds. The UV–visible absorption bands were assigned as mainly ligand-centered or metal-to-ligand charge-transfer transitions, with strong contributions from the chlorido and azido groups. In complexes with substituted Ph-azpy ligands, σ-donation from NMe2 and OH/O– groups results in a redshift of the main absorption bands compared with unsubstituted Ph-azpy complexes. The diazido complexes are photoactive in solution upon irradiation with either UVA or visible light for R = H or NMe2, or UVA only when R = OH/O–. Intriguingly, the phenolate group of the latter complex undergoes very slow protonation in solution. Biological screening was limited by poor solubility; however, initial tests showed that the phenolato diazido complex is rapidly taken up into the nuclei of HaCaT keratinocytes, which are stained intensely blue, and its cytotoxicity is increased upon irradiation with UVA light.
中文翻译:

光活性铂 (II) 偶氮吡啶配合物†
铂 (II) 配合物含有强 π-受体N , N - 螯合配体 苯基偶氮吡啶 (Ph-azpy) [Pt( p -R-Ph-azpy)X 2 ], R = H, NMe 2或 OH, X = Cl或 N 3, 已经合成和表征,以探索单齿配体和苯基取代基对其吸收光谱和光活化的影响。时间相关的密度泛函理论计算表明,配合物具有低空未占轨道,对大多数配位键具有较强的 σ 反键特性。紫外可见吸收带主要被指定为以配体为中心或金属到配体的电荷转移跃迁,其中氯代和叠氮基的贡献很大。在具有取代的 Ph-azpy 配体的配合物中,来自 NMe 2和 OH/O的 σ-捐赠–与未取代的 Ph-azpy 配合物相比,基团导致主要吸收带的红移。对于 R = H 或 NMe 2,当用 UVA 或可见光照射时,二叠氮基配合物在溶液中具有光活性,或者仅当 R = OH/O -时才使用 UVA 。有趣的是,后一种配合物的酚盐基团在溶液中经历了非常缓慢的质子化。生物筛选受到溶解度差的限制;然而,最初的测试表明,二叠氮苯酚复合物被迅速吸收到 HaCaT 角质形成细胞的细胞核中,这些细胞核被染成深蓝色,并且在 UVA 光照射下其细胞毒性增加。
更新日期:2021-02-22
中文翻译:

光活性铂 (II) 偶氮吡啶配合物†
铂 (II) 配合物含有强 π-受体N , N - 螯合配体 苯基偶氮吡啶 (Ph-azpy) [Pt( p -R-Ph-azpy)X 2 ], R = H, NMe 2或 OH, X = Cl或 N 3, 已经合成和表征,以探索单齿配体和苯基取代基对其吸收光谱和光活化的影响。时间相关的密度泛函理论计算表明,配合物具有低空未占轨道,对大多数配位键具有较强的 σ 反键特性。紫外可见吸收带主要被指定为以配体为中心或金属到配体的电荷转移跃迁,其中氯代和叠氮基的贡献很大。在具有取代的 Ph-azpy 配体的配合物中,来自 NMe 2和 OH/O的 σ-捐赠–与未取代的 Ph-azpy 配合物相比,基团导致主要吸收带的红移。对于 R = H 或 NMe 2,当用 UVA 或可见光照射时,二叠氮基配合物在溶液中具有光活性,或者仅当 R = OH/O -时才使用 UVA 。有趣的是,后一种配合物的酚盐基团在溶液中经历了非常缓慢的质子化。生物筛选受到溶解度差的限制;然而,最初的测试表明,二叠氮苯酚复合物被迅速吸收到 HaCaT 角质形成细胞的细胞核中,这些细胞核被染成深蓝色,并且在 UVA 光照射下其细胞毒性增加。



























 京公网安备 11010802027423号
京公网安备 11010802027423号