Water Research ( IF 12.8 ) Pub Date : 2021-02-18 , DOI: 10.1016/j.watres.2021.116951 Xian-Shi Wang , Heng Song , Jing Zhang , Yu-Lei Liu , Jun Ma , Lu Wang
|
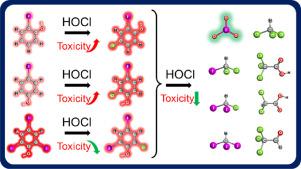
Highly toxic iodinated phenolic by-products were frequently detected in the oxidative treatment and disinfection of iodine-containing water. Herein, it was found that three model iodinated phenolic disinfection byproducts (DBPs), 2-iodophenol, 4-iodophenol and 2,4,6-triiodophenol, were reactive with HOCl, and the reaction rate constants (at pH 7.0 and 25℃) were 1.86 ×102, 1.62 ×102 and 7.5 ×101 M−1s−1, respectively. When HOCl was in excess (HOCl/iodophenol = 40/1, [iodophenol]0 = 20 μM), acute toxicity of water sample containing iodophenols could be largely eliminated (> 85%), with the conversion of iodophenols into stable and non-toxic iodate (IO3−) and iodinated and chlorinated aliphatic DBPs. Besides IO3−, seven kinds of aromatic intermediate products including iodophenols, chloroiodophenols, iodoquinones, chloroiodoquinones, chloroquinones, chlorophenols, and coupling products were detected. C-I bond of iodophenols was cleaved in the reaction and the resulted aromatic products were further transformed into chlorinated aliphatic DBPs [trichloromethane (TCM), trichloroacetic acid (TCAA), dichloroacetic acid (DCAA), and chloral hydrate (CH)] (mg/L level) and iodinated trihalomethanes (μg/L level). HOCl was effective for converting iodophenols into IO3− and less toxic chlorinated aliphatic DBPs. Considering that chlorine was widely used as disinfectant, transformation and toxicity alteration of emerging DBPs during chlorination/booster chlorination warrant further investigations.
中文翻译:

氯化通过形成碘酸盐和氯化脂肪族消毒副产物而降低了碘酚的急性毒性。
在含碘水的氧化处理和消毒中经常检测到剧毒的碘化酚类副产物。在此发现,三种模型碘化酚醛消毒副产物(2-碘苯酚,4-碘苯酚和2,4,6-三碘苯酚)可与HOCl反应,且反应速率常数(在pH 7.0和25℃下)分别为1.86×10 2,1.62×10 2至7.5×10 1中号-1小号-1,分别。当HOCl过量时(HOCl /碘苯酚= 40/1,[碘苯酚] 0 = 20μM),含有碘苯酚的水样品的急性毒性可以大大消除(> 85%),并将碘苯酚转化为稳定的和非碘的。有毒的碘酸盐(IO 3 -)以及碘化和氯化的脂肪族DBP。除了IO 3 - ,检测到7种芳香族中间产品,包括iodophenols,chloroiodophenols,iodoquinones,chloroiodoquinones,chloroquinones,氯酚,和偶联产物。碘酚的CI键在反应中裂解,所得芳族产物进一步转化为氯化脂肪族DBP [三氯甲烷(TCM),三氯乙酸(TCAA),二氯乙酸(DCAA)和水合氯醛(CH)](mg / L含量)和碘代三卤甲烷(μg/ L含量)。次氯酸是有效的转化成iodophenols IO 3 -和毒性较小的氯化脂肪族DBP。考虑到氯已被广泛用作消毒剂,因此在加氯/加强氯化过程中,新出现的DBP的转化和毒性改变值得进一步研究。


























 京公网安备 11010802027423号
京公网安备 11010802027423号