Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gold leaf electrochemical sensors: applications and nanostructure modification
Analyst ( IF 4.2 ) Pub Date : 2021-2-10 , DOI: 10.1039/d0an02455d Paithoon Prasertying 1, 2, 3, 4, 5 , Nanthatchaphon Jantawong 1, 2, 3, 4, 5 , Thitaporn Sonsa-ard 1, 2, 3, 4, 5 , Thinnapong Wongpakdee 1, 2, 3, 4, 5 , Nuttamon Khoonrueng 1, 2, 3, 4, 5 , Supatana Buking 6, 7, 8, 9 , Duangjai Nacapricha 1, 2, 3, 4, 5
Analyst ( IF 4.2 ) Pub Date : 2021-2-10 , DOI: 10.1039/d0an02455d Paithoon Prasertying 1, 2, 3, 4, 5 , Nanthatchaphon Jantawong 1, 2, 3, 4, 5 , Thitaporn Sonsa-ard 1, 2, 3, 4, 5 , Thinnapong Wongpakdee 1, 2, 3, 4, 5 , Nuttamon Khoonrueng 1, 2, 3, 4, 5 , Supatana Buking 6, 7, 8, 9 , Duangjai Nacapricha 1, 2, 3, 4, 5
Affiliation
|
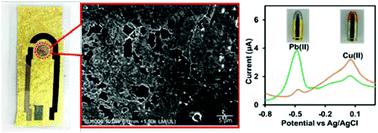
This work presents the first planar three-electrode electrochemical sensor comprising local gold leaf as the working electrode and printed, or hand-drawn, counter and reference electrodes, respectively. The gold leaf was mounted on a polyvinyl chloride (PVC) adhesive sheet (15 mm × 30 mm) and covered with a second PVC sheet printed with the counter and reference electrodes. This sheet has a 3 mm circle and a 2 mm × 3 mm rectangle removed to expose the gold electrode area and electrical contacts, respectively. A third shorter insulating layer with a 10 mm circular hole was placed on top to delineate the sensing area of all electrodes. The sensor displayed expected performances in various modes of operation, such as cyclic voltammetry, square wave voltammetry and anodic stripping voltammetry. For the latter mode, the limit of detection of Pb(II) was 3.2 μg L−1, compliant with regulation for drinking water (10 μg L−1 Pb(II)). Although designed as a disposable unit, the electrode is effective for up to 200 cycles and applicable for multiple use. The gold leaf was modified by electrodeposition of the gold network and large nano-size gold particles which significantly enhanced the sensitivity of all voltametric sensing, giving lower limits of detection. For stripping voltammetry, the electroplating structure modification improved the simultaneous detection of lead and copper, with the copper response increasing 6-fold. The device has the capability of on-site identification of copper/lead bullets from gunshot residues within 6 min.
中文翻译:

金箔电化学传感器:应用和纳米结构修饰
这项工作提出了第一个平面三电极电化学传感器,该传感器包括局部金箔作为工作电极,并分别印刷或手绘对电极和参比电极。将金箔固定在聚氯乙烯(PVC)粘合片(15毫米×30毫米)上,并用印有对电极和参比电极的第二张PVC片覆盖。该薄片的3mm圆和2mm×3mm的矩形被去掉,分别露出金电极区域和电触点。将具有10 mm圆形孔的第三较短绝缘层放在顶部,以描绘所有电极的感应区域。该传感器在各种操作模式下均显示出预期的性能,例如循环伏安法,方波伏安法和阳极溶出伏安法。对于后一种模式,Pb(II)为3.2μgL -1,符合饮用水法规(10μgL -1 Pb(II))。尽管设计为一次性装置,但该电极最多可有效循环200次,并且可多次使用。金箔通过电沉积金网络和较大的纳米级金颗粒进行修饰,从而显着增强了所有伏安传感的灵敏度,从而降低了检测限。对于溶出伏安法,电镀结构的改进改善了对铅和铜的同时检测,铜响应增加了6倍。该设备能够在6分钟内现场识别枪击残留物中的铜/铅子弹。
更新日期:2021-02-18
中文翻译:

金箔电化学传感器:应用和纳米结构修饰
这项工作提出了第一个平面三电极电化学传感器,该传感器包括局部金箔作为工作电极,并分别印刷或手绘对电极和参比电极。将金箔固定在聚氯乙烯(PVC)粘合片(15毫米×30毫米)上,并用印有对电极和参比电极的第二张PVC片覆盖。该薄片的3mm圆和2mm×3mm的矩形被去掉,分别露出金电极区域和电触点。将具有10 mm圆形孔的第三较短绝缘层放在顶部,以描绘所有电极的感应区域。该传感器在各种操作模式下均显示出预期的性能,例如循环伏安法,方波伏安法和阳极溶出伏安法。对于后一种模式,Pb(II)为3.2μgL -1,符合饮用水法规(10μgL -1 Pb(II))。尽管设计为一次性装置,但该电极最多可有效循环200次,并且可多次使用。金箔通过电沉积金网络和较大的纳米级金颗粒进行修饰,从而显着增强了所有伏安传感的灵敏度,从而降低了检测限。对于溶出伏安法,电镀结构的改进改善了对铅和铜的同时检测,铜响应增加了6倍。该设备能够在6分钟内现场识别枪击残留物中的铜/铅子弹。


























 京公网安备 11010802027423号
京公网安备 11010802027423号