当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deconvolution of intermixed redox processes in Ni-based cation-disordered Li-excess cathodes
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2021-2-2 , DOI: 10.1039/d0ee03526b Tzu-Yang Huang 1, 2, 3, 4, 5 , Matthew J. Crafton 1, 2, 3, 4, 5 , Yuan Yue 3, 4, 5, 6 , Wei Tong 3, 4, 5, 6 , Bryan D. McCloskey 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2021-2-2 , DOI: 10.1039/d0ee03526b Tzu-Yang Huang 1, 2, 3, 4, 5 , Matthew J. Crafton 1, 2, 3, 4, 5 , Yuan Yue 3, 4, 5, 6 , Wei Tong 3, 4, 5, 6 , Bryan D. McCloskey 1, 2, 3, 4, 5
Affiliation
|
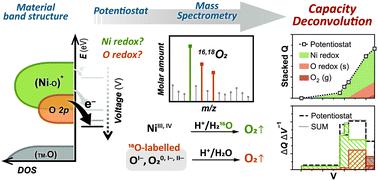
Cation-disordered rock-salt transition-metal oxides and oxyfluorides (DRX) have emerged as promising cathode materials for Li-ion batteries due to their potential to reach high energy densities and accommodate diverse, lower cost transition-metal chemistries compared to conventional layered oxide materials. However, the intricate local coordination environment in DRX also results in complex electrochemical electron transfer involving parallel mechanisms of transition-metal (TM) redox and oxygen (anionic) redox. Without decoupled and quantitative information of these intermixed redox processes, the origin of irreversibility, voltage hysteresis, and capacity fading is obscured, impeding the development of strategies to address these issues. Here we deconvolute the mixed redox processes in a Ni-based DRX, Li1.15Ni0.45Ti0.3Mo0.1O1.85F0.15, by combining 18O isotopic enrichment, differential electrochemical mass spectrometry (DEMS), and ex situ acid titration. The summation of TM-redox and oxygen-redox capacities measured through our approach agrees with the net electron transfer measured by the potentiostat. This study reveals much less Ni oxidation efficiency (59.5%) than its initially designed efficiency (100%) due to competition of oxygen redox, which can occur at potentials as low as 4.1 V (vs. Li/Li+). We propose that the chemical approach presented in this work and its future extension can resolve and quantify various mixed redox processes in different DRX, which allows clear correlations among material design, deconvoluted redox capacities, and battery performance.
中文翻译:

镍基阳离子无序锂过量阴极中混合氧化还原过程的反卷积
阳离子无序的岩盐过渡金属氧化物和氟氧化物(DRX)已成为锂离子电池的有希望的正极材料,因为与传统的层状氧化物相比,它们具有达到高能量密度并适应各种低成本的过渡金属化学的潜力材料。但是,DRX中复杂的局部配位环境也导致复杂的电化学电子转移,涉及过渡金属(TM)氧化还原和氧(阴离子)氧化还原的平行机理。如果没有这些混合的氧化还原过程的解耦和定量信息,则不可逆性,电压滞后和容量衰减的根源就被掩盖了,从而阻碍了解决这些问题的策略的发展。在这里,我们对镍基DRX Li 1.15中的混合氧化还原过程进行反卷积Ni 0.45 Ti 0.3 Mo 0.1 O 1.85 F 0.15,通过18 O同位素富集,差分电化学质谱(DEMS)和异位酸滴定相结合。通过我们的方法测得的TM-氧化还原和氧-氧化还原容量的总和与恒电位仪测得的净电子转移一致。这项研究表明,由于氧气氧化还原的竞争,镍的氧化效率(59.5%)比其最初设计的效率(100%)低得多,而氧化还原可以在低至4.1 V的电势下发生(vs. Li / Li +)。我们建议,这项工作及其未来扩展中介绍的化学方法可以解析和量化不同DRX中的各种混合氧化还原过程,从而可以在材料设计,去卷积的氧化还原容量和电池性能之间建立明确的关联。
更新日期:2021-02-17
中文翻译:

镍基阳离子无序锂过量阴极中混合氧化还原过程的反卷积
阳离子无序的岩盐过渡金属氧化物和氟氧化物(DRX)已成为锂离子电池的有希望的正极材料,因为与传统的层状氧化物相比,它们具有达到高能量密度并适应各种低成本的过渡金属化学的潜力材料。但是,DRX中复杂的局部配位环境也导致复杂的电化学电子转移,涉及过渡金属(TM)氧化还原和氧(阴离子)氧化还原的平行机理。如果没有这些混合的氧化还原过程的解耦和定量信息,则不可逆性,电压滞后和容量衰减的根源就被掩盖了,从而阻碍了解决这些问题的策略的发展。在这里,我们对镍基DRX Li 1.15中的混合氧化还原过程进行反卷积Ni 0.45 Ti 0.3 Mo 0.1 O 1.85 F 0.15,通过18 O同位素富集,差分电化学质谱(DEMS)和异位酸滴定相结合。通过我们的方法测得的TM-氧化还原和氧-氧化还原容量的总和与恒电位仪测得的净电子转移一致。这项研究表明,由于氧气氧化还原的竞争,镍的氧化效率(59.5%)比其最初设计的效率(100%)低得多,而氧化还原可以在低至4.1 V的电势下发生(vs. Li / Li +)。我们建议,这项工作及其未来扩展中介绍的化学方法可以解析和量化不同DRX中的各种混合氧化还原过程,从而可以在材料设计,去卷积的氧化还原容量和电池性能之间建立明确的关联。



























 京公网安备 11010802027423号
京公网安备 11010802027423号